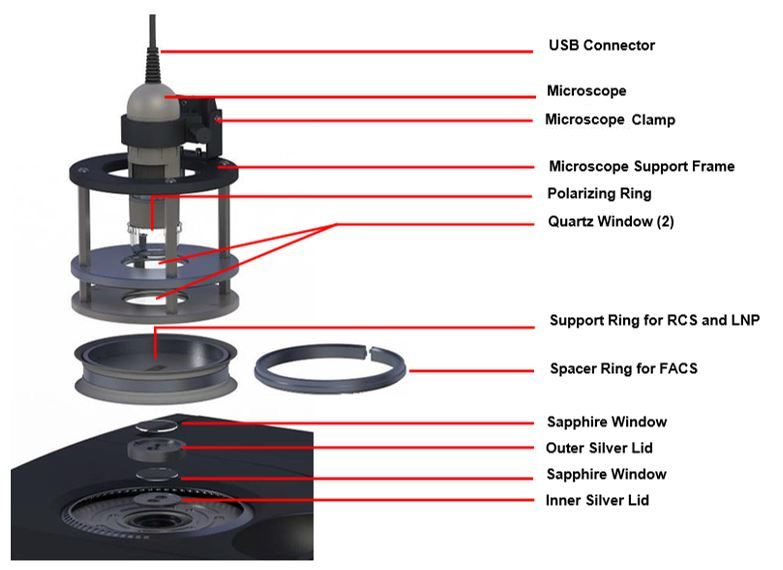
Microscope Accessory
DSC performed with the microscope accessory allows the scientist to capture images and video of:
- Crystallization and melt transitions
- Flow of materials above the glass transition and melting point
- Volumetric, dimensional changes associated with a phase transition
- Visual observations of color and other changes in appearance
DSC Microscopy can be utilized by the chemical materials, food, pharmaceutical and inorganic industries.
Discovery DSC Microscope Accessory:
- Includes a high resolution (1.3 MP vivid color) digital microscope camera; typical magnification of 50-65X when focused on a sample pan in the DSC cell (~4-5mm field of view)
- Collects individual images up to 1 fps (frames per second) and video at 15 fps
- Illumination provided by several white light LEDs and an adjustable polarizer is included for viewing highly reflective materials and minimizing the amount of glare
DSC performed with the microscope accessory has several applications that may prove insightful to better understanding materials’ properties and behaviors.
It allows the scientist to capture images of:
- crystallization and melt transitions
- flow of materials above the glass transition and melting point
- volumetric, dimensional changes associated with a phase transition
- visual observations of coloration and other deviations in appearance
| Specifications and Requirements | |
| DSC Compatibility | DSC 2500, 250 and 25 |
| Cooler Compatibility | LN pump, RCS 120/90/40, FACS |
| Operational Temperature Range | -180 to 725°C |
| Microscope Specifications | Resolution 1080 x 1024 pixels
>40X zoom (50X-65X optimal, 90X max) Monochrome and color still images Video with polarizer Compatibility with Windows 7, 8 and 10 USB 2.0 port connected to the controller computer |
| Software | TRIOS software
Enabled method segments trigger image and video capture with accurate image-time superposition 15 frames/sec image acquisition rate (video) 1 frame/sec image acquisition rate (images) Images easily exported from data file within TRIOS |
| Gas Compatibility | Nitrogen, helium, argon, air and oxygen |
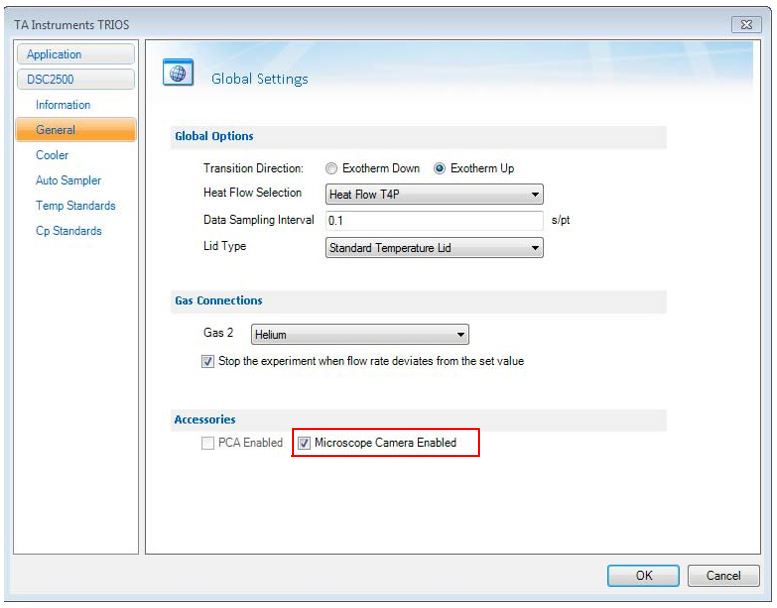
Selecting the Microscope Camera Accessory in TRIOS Options will:
- Automatically park and then disable the autosampler
- Activate the Microscope method segments
Available Method Segments:
- Image: This segment allows the user to turn the image collection command on and off during an experiment. The images are stored within the TRIOS data file and can be exported into standard formats such as *.jpg
- Video: This segment allows the user to turn the video collection command on and off during an experiment. The video file is not embedded in the TRIOS data file; it is a separate file stored in the same directory as the TRIOS data file. The video file is saved as *.avi
- Dynamic Video: This segment is used to capture video clips only when a heat flow thermal event in the test sample is detected. This helps reduce the size of the video footage by not capturing video during inactive heat flow time periods. The heat flow transition detection trigger initiates when the heat flow rate acceleration is detected to be approximately 0.1 mW/sec2. At this point, a video clip is created that contains up to 20 seconds of pre-transition footage, along with footage during the transition
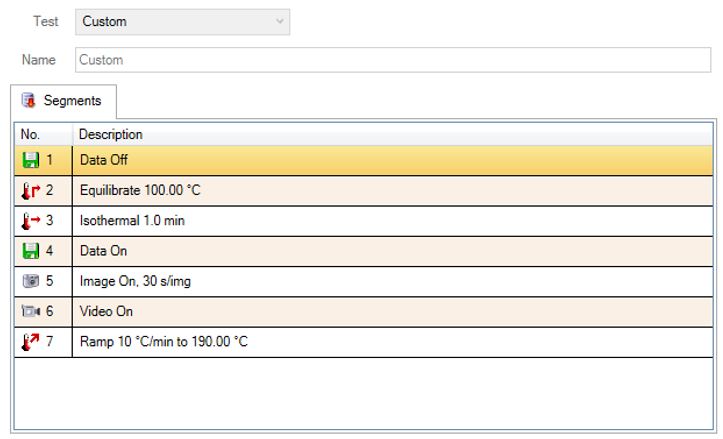
A Custom method with Image and Video segments.
A 5 minute isothermal segment can be included at the start of the test to pre-purge the cell to remove residential air prior to sub-ambient testing.
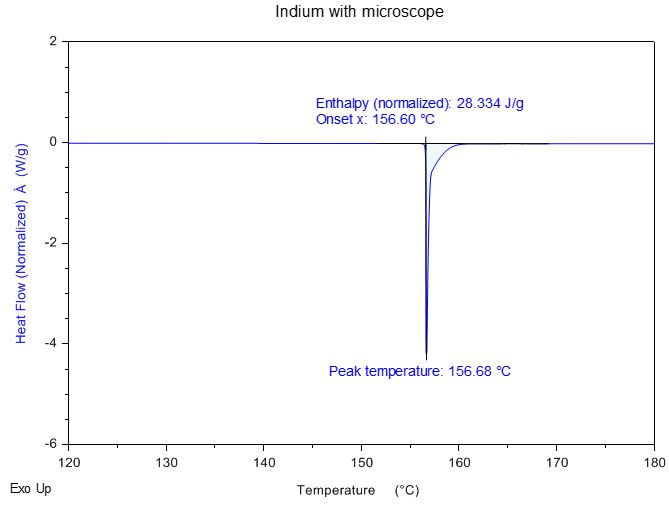
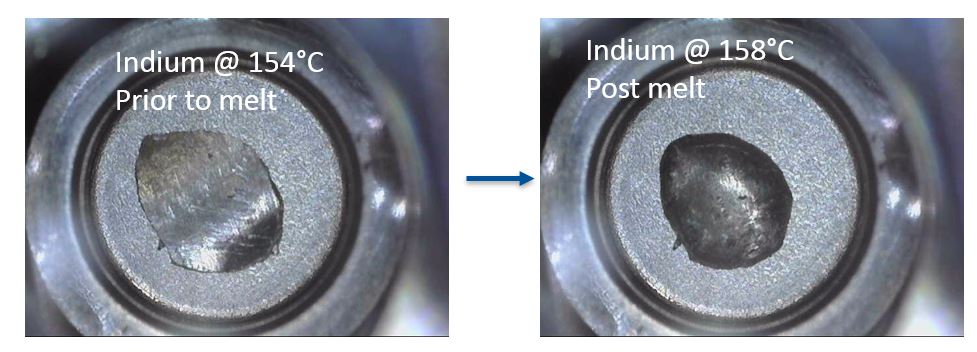
Indium
Indium
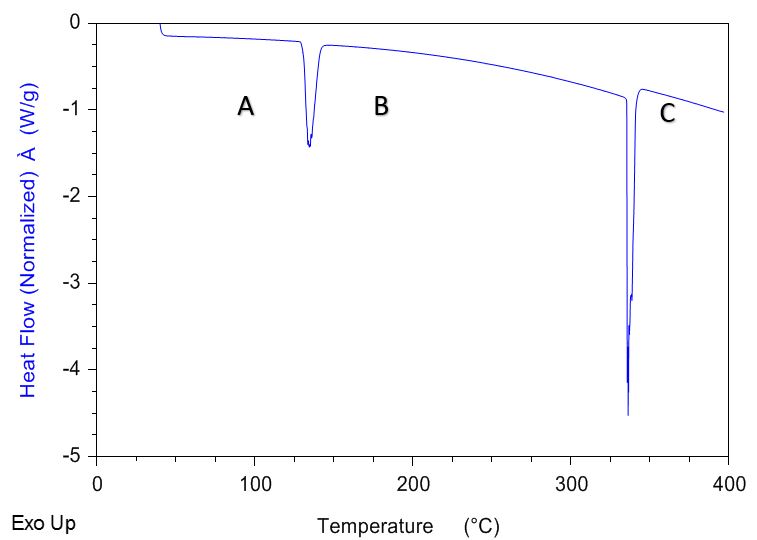
Potassium Nitrate
Potassium Nitrate
- Potassium Nitrate, KNO3, is an inorganic salt with the appearance of a white solid (powder)
- A standard temperature ramp to 400°C (below the decomposition temperature) demonstrates that KNO3 exhibits two endothermic events
- Event 1 @ 130°C: A solid-solid conversion from an orthorhombic crystal structure to trigonal
- Event 2 @ 334°C: Melt transition

Polylactic Acid
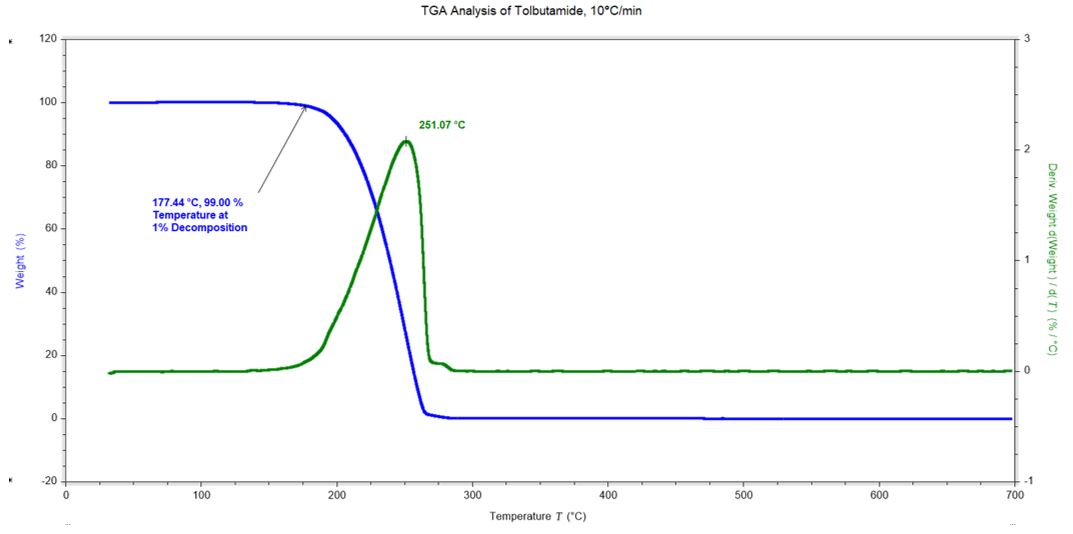
Tests performed with the DSC microscope accessory use unsealed, open DSC pans. It is important to understand the thermal stability of the material being tested to minimize contamination in the DSC cell.
Weight changes in a material may be due to:
- Evaporation of solvents
- Sublimation
- Decomposition
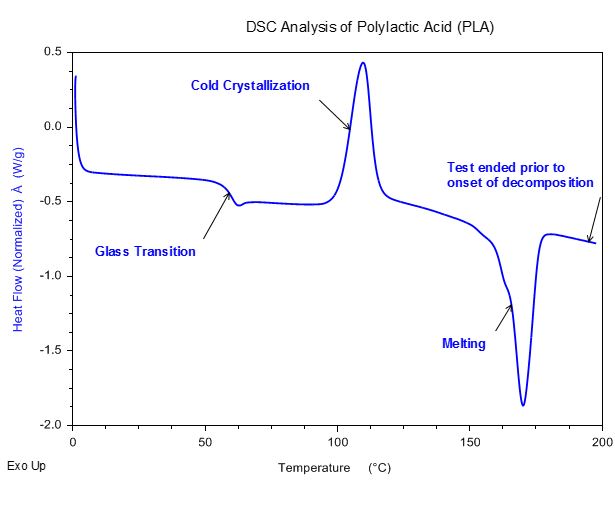
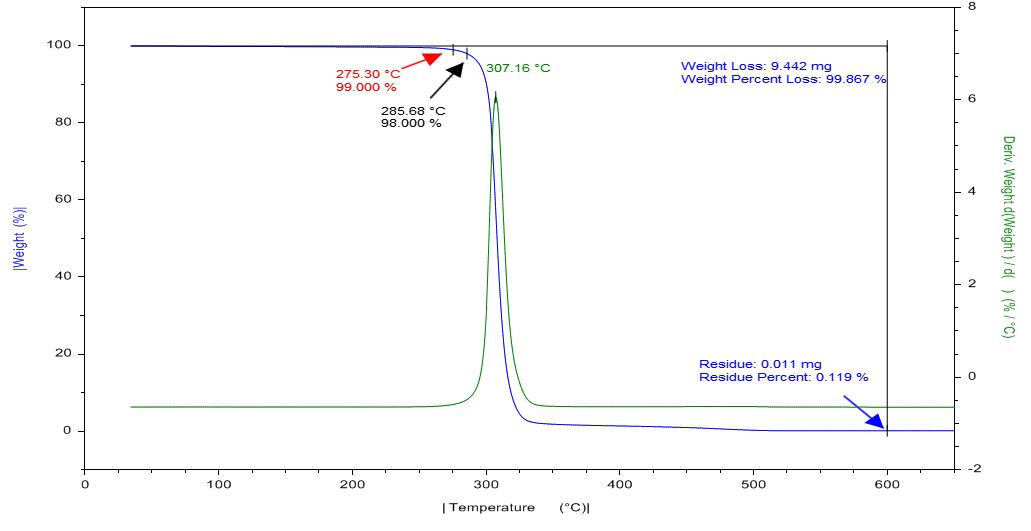
DSC Analysis of Polylactic Acid (PLA)
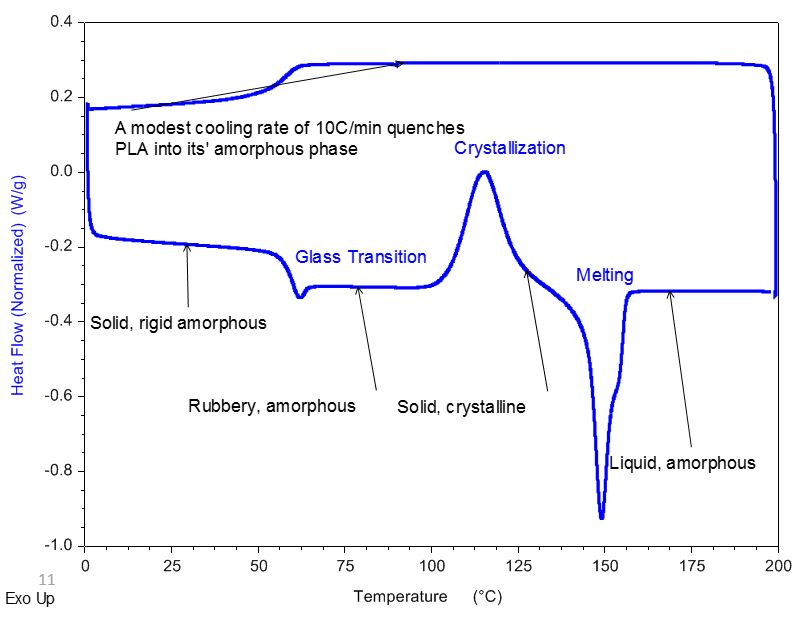
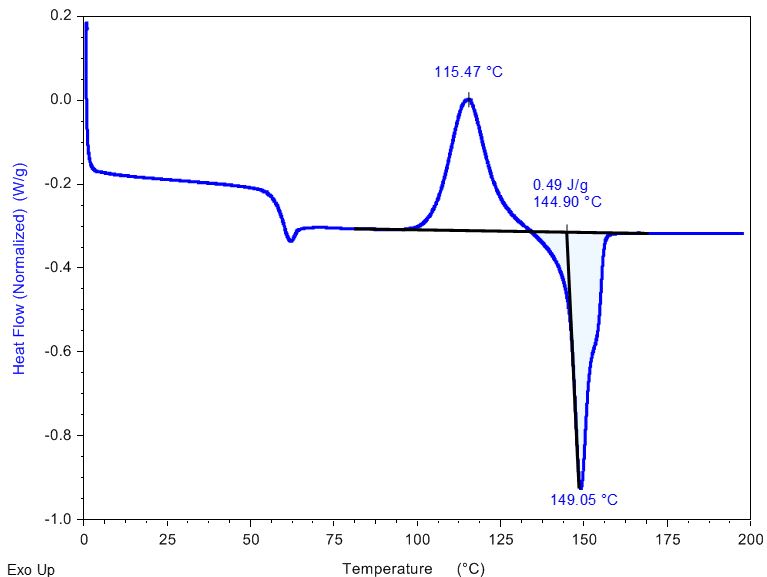
DSC Analysis of Polylactic Acid (PLA): Pre-Tg
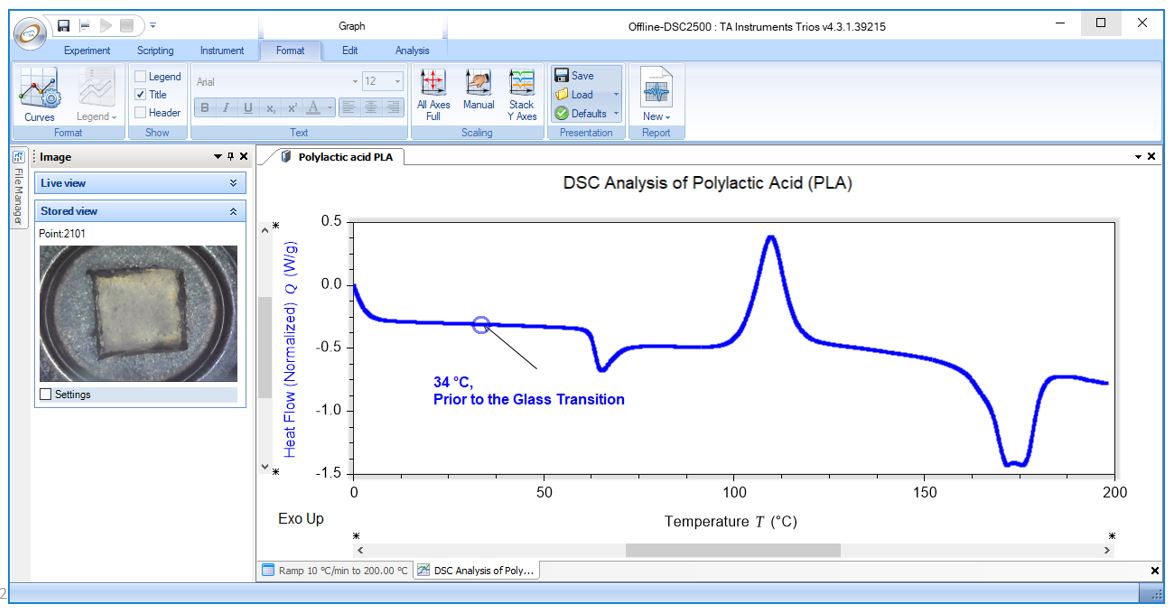
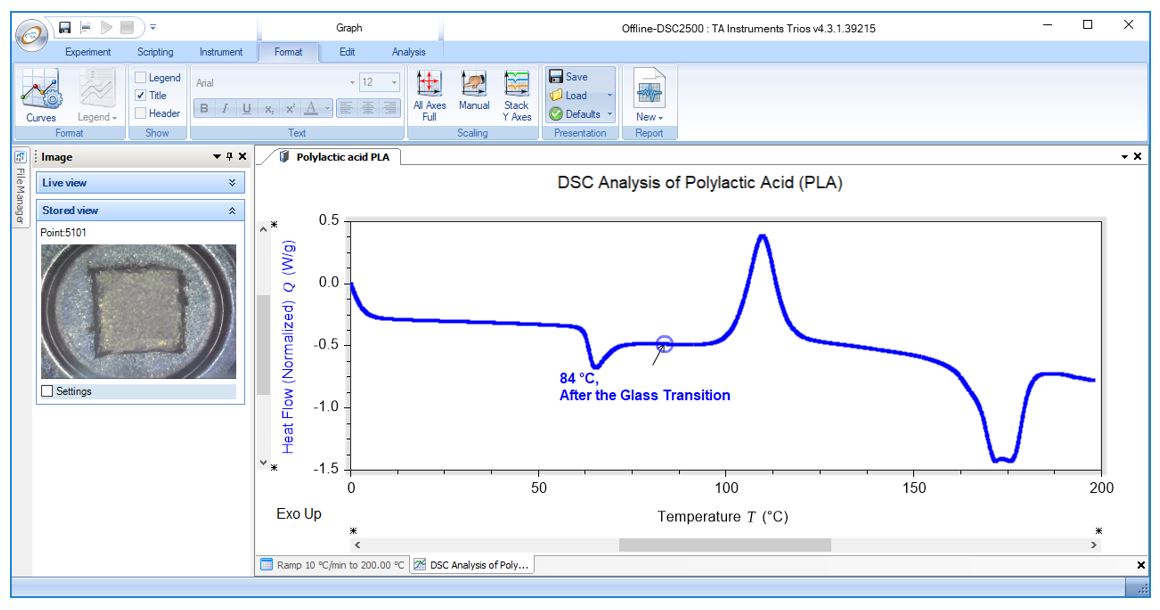
DSC Analysis of Polylactic Acid (PLA): Post-Tg
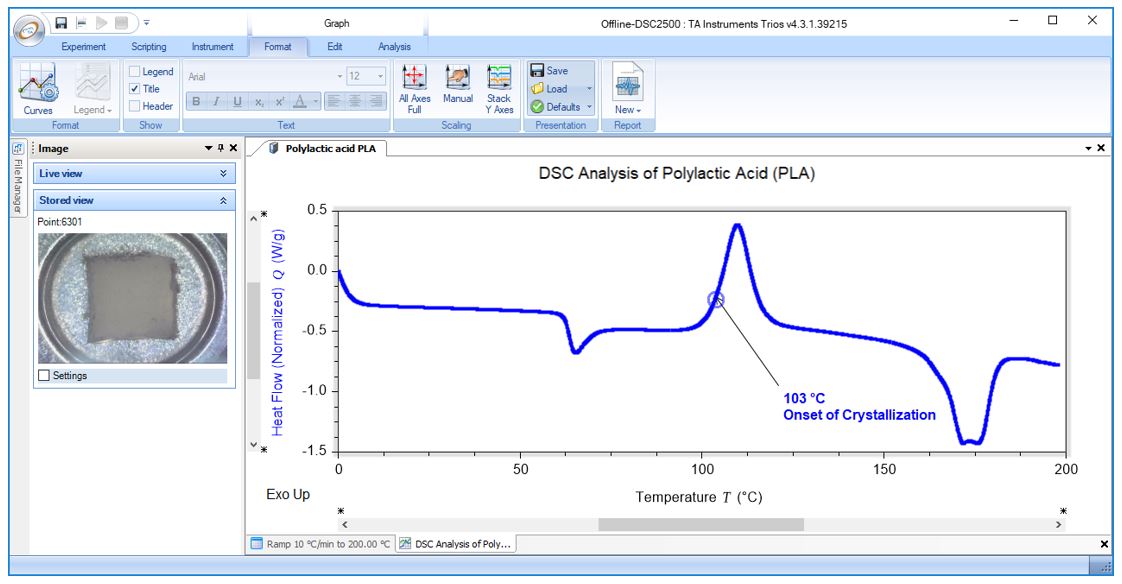
DSC Analysis of Polylactic Acid (PLA): Onset of Crystallization
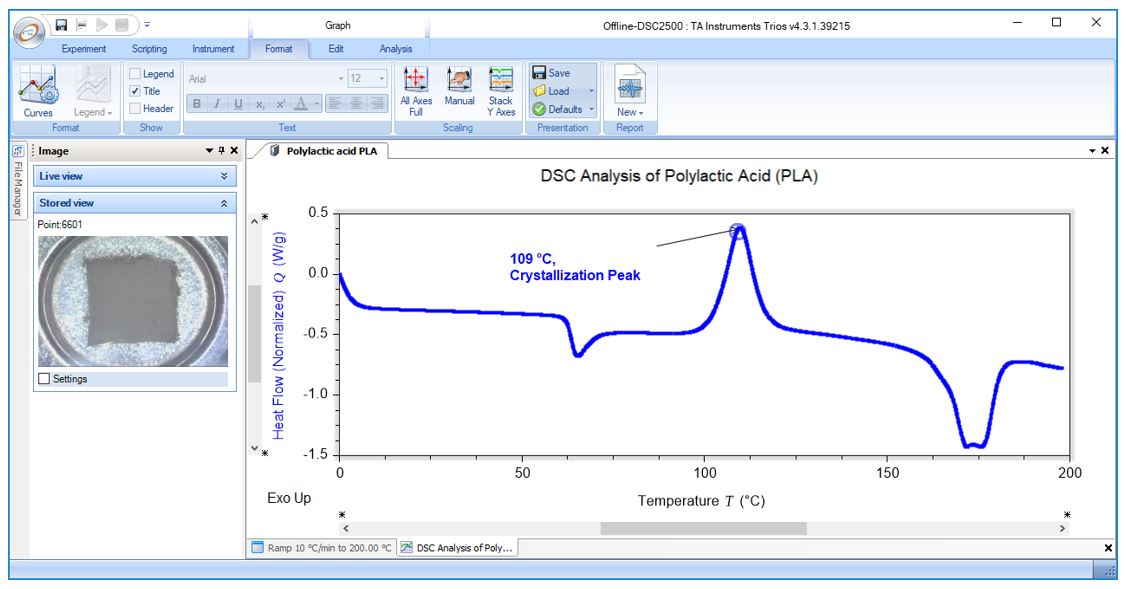
DSC Analysis of Polylactic Acid (PLA): Crystallization Peak
DSC Analysis of Polylactic Acid (PLA): Melting
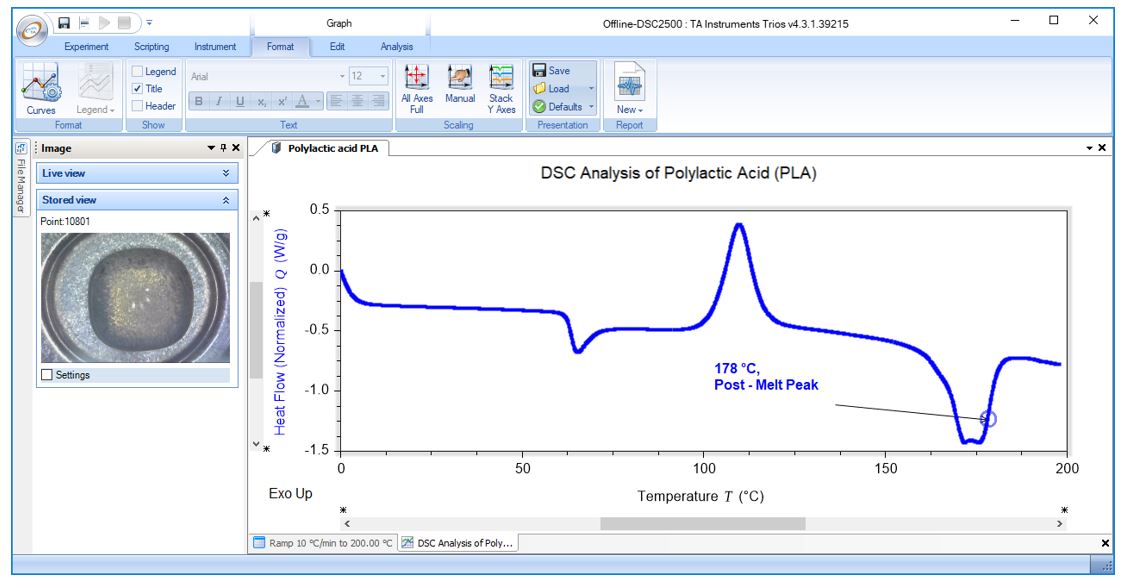
DSC Analysis of Polylactic Acid (PLA): Post-Melt
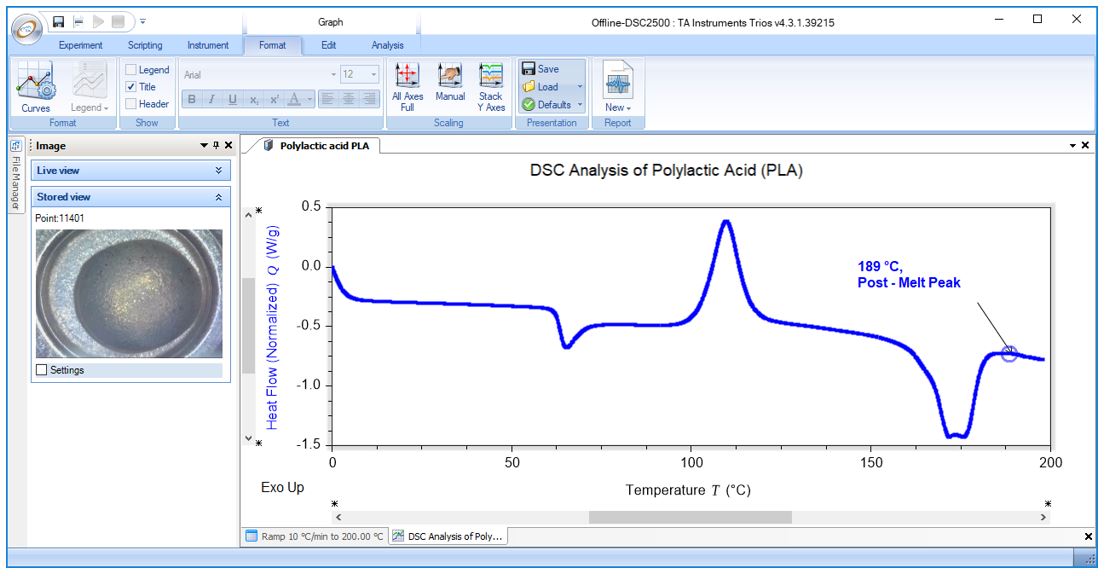
Thermal Analysis of Pharmaceutical Polymorphs
- Polymorphism: the ability of the same chemical structure to exist in multiple crystal forms called polymorphs
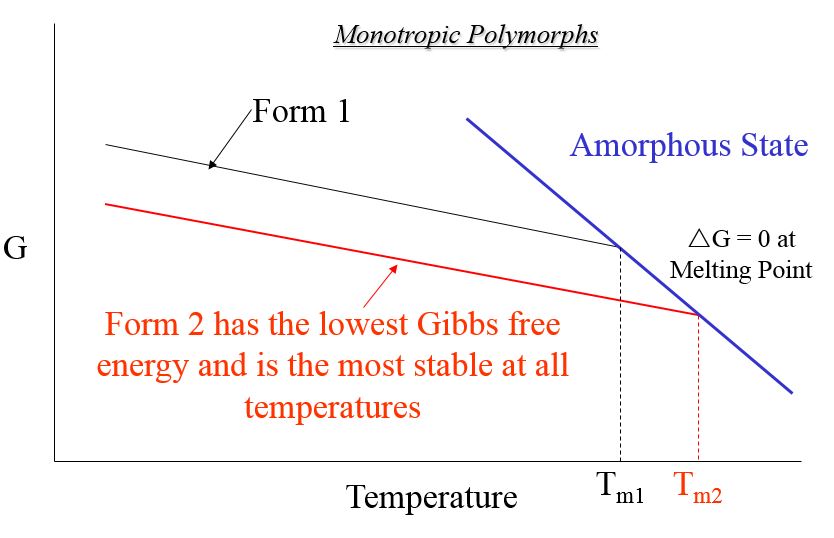
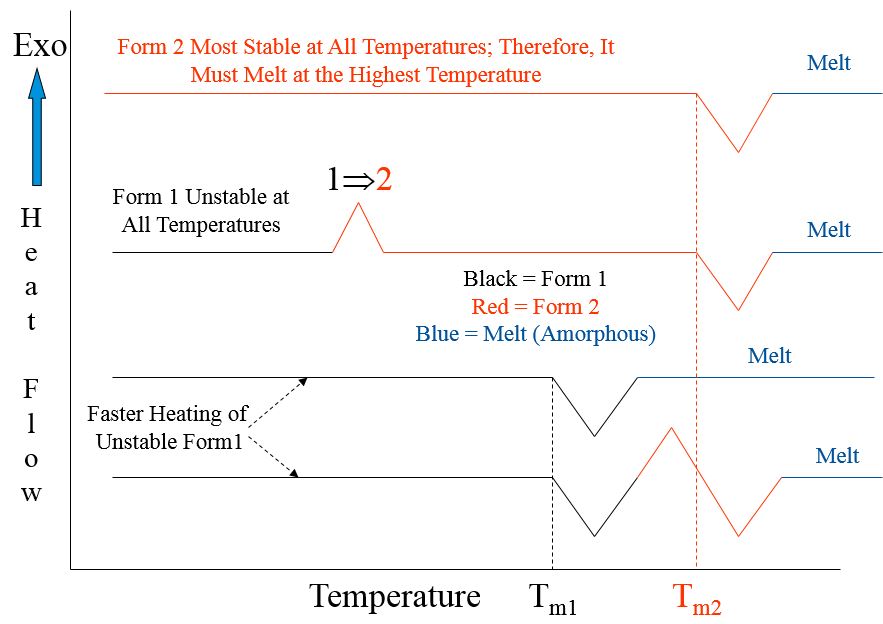
- Monotropic Polymorphism: one crystal structure (polymorph) is the most stable at all temperatures
- Enantiotropic Polymorphism: different polymorphs are the most stable over specific temperature ranges
- The chemistry is the same but the form of the drug affects:
- Solubility
- Dissolution Rate
- Bioavailability
- Formulation Development
- Drug-excipient incompatibility
- Manufacturability
- Storage Stability
The Most Stable Polymorph Has the Lowest;
- Gibbs Free Energy; ΔG = ΔH – TΔS
- Solubility in any solvent
- Dissolution rate
- Bioavailability
- Reactivity
Most Stable Polymorph Has the Highest;
- Melting Point (Tm) only if * the system is Monotropic
- Latent Heat of Melting (rHm) only if * the system is Monotropic
*Therefore, melting point or heat of melting can not be used directly to predict polymorphic stability. It is necessary to create a relative Gibbs Free Energy Plot
Relative Gibbs Free Energy Plot for Monotropic Polymorphic Crystal Forms
Relative Gibbs Free Energy Plot for Monotropic Polymorphic Crystal Forms
Possible DSC Results on Monotropic Polymorphs (Endothermic Solid-Solid Transformation Not Possible)
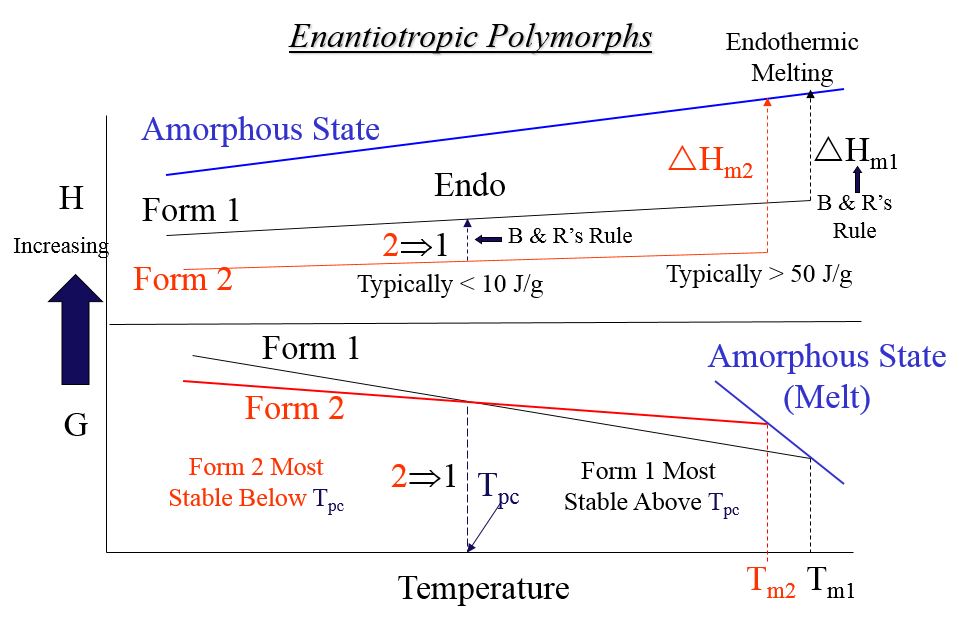
Relative Gibbs Free Energy Plot for Enantiotropic Polymorphic Crystal Forms
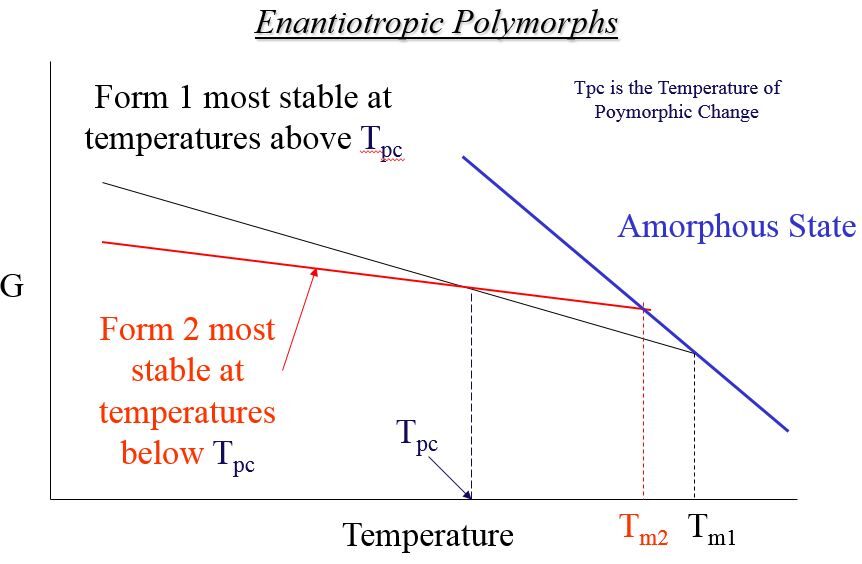
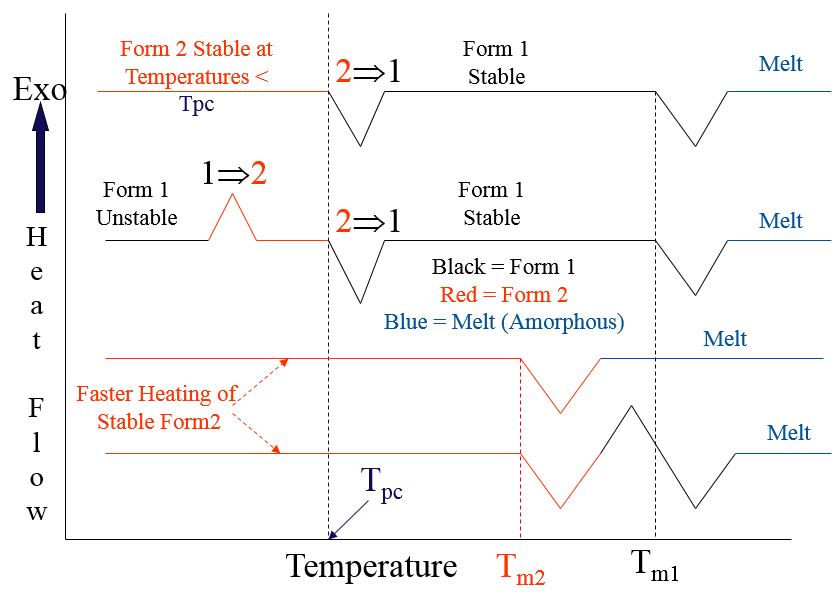
Possible DSC Results on Enantiotropic Polymorphs
DSC Analysis of Tolbutamide – Enantiotropic Polymorphs Pharmaceutical drug used to manage type 2 diabetes
DSC Analysis of Tolbutamide – Enantiotropic Polymorphs Pharmaceutical drug used to manage type 2 diabetes
Burger and Ramberger’s Rules
Burger and Ramberger’s Rules
Burger and Ramberger’s Rules (important to identify enantiotropic polymorphs)
- Heat of Transition Rule; if an endothermic transition (from one solid form to another) is observed, the two forms are enantiotropic
- Heat of Fusion Rule; if the higher melting form has a lower heat of melting, the two forms are enantiotropic
Illustrating Burger and Ramberger’s Rules
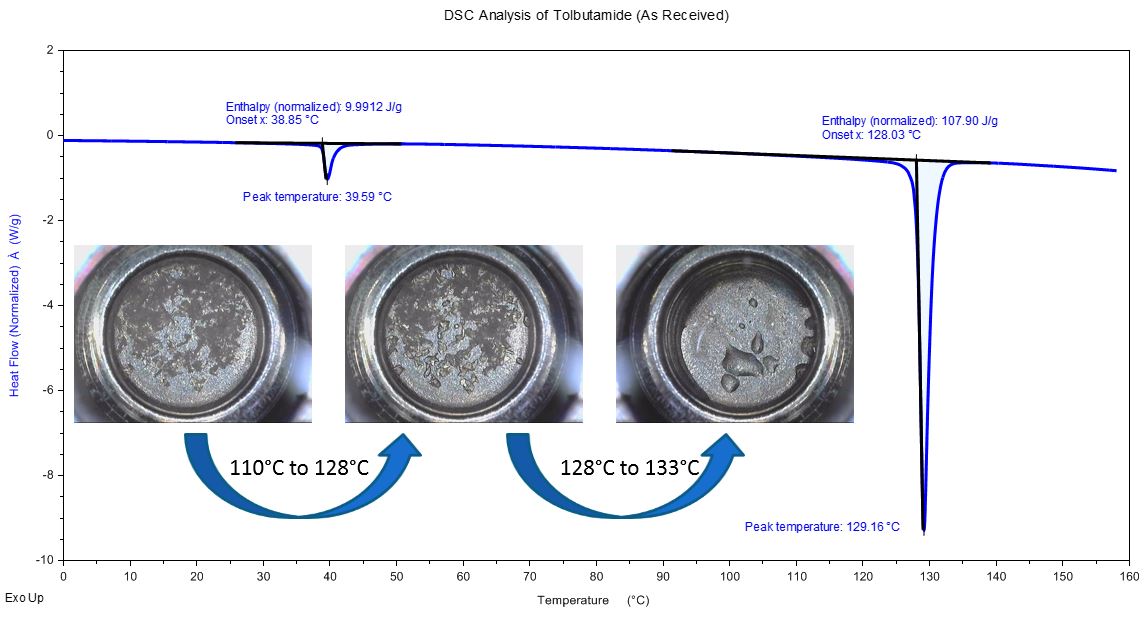
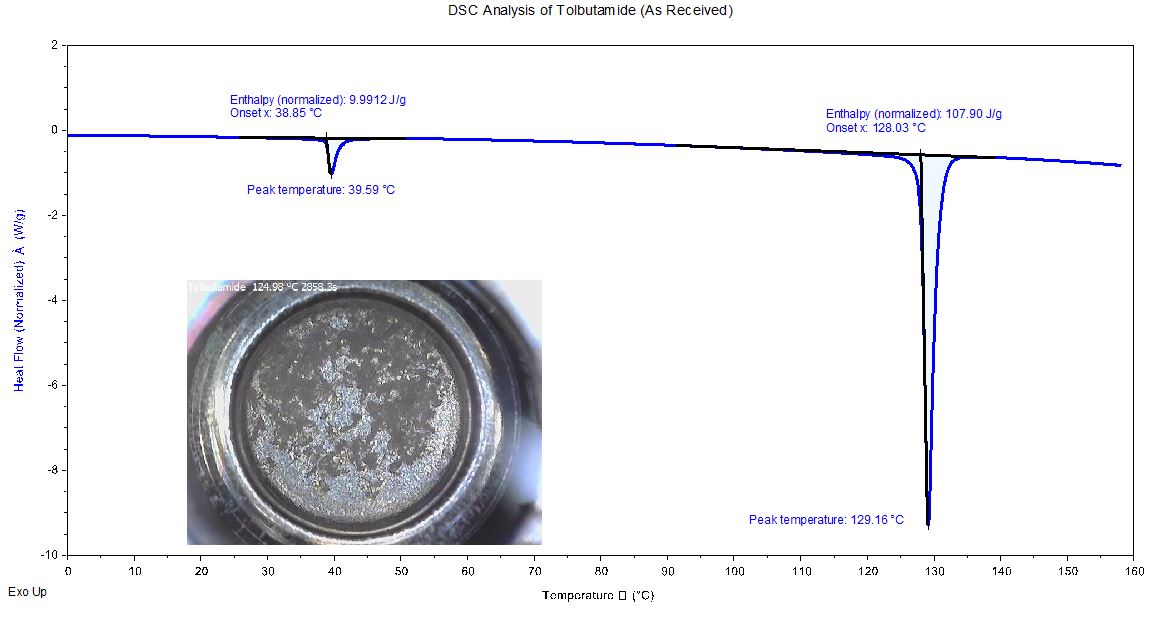
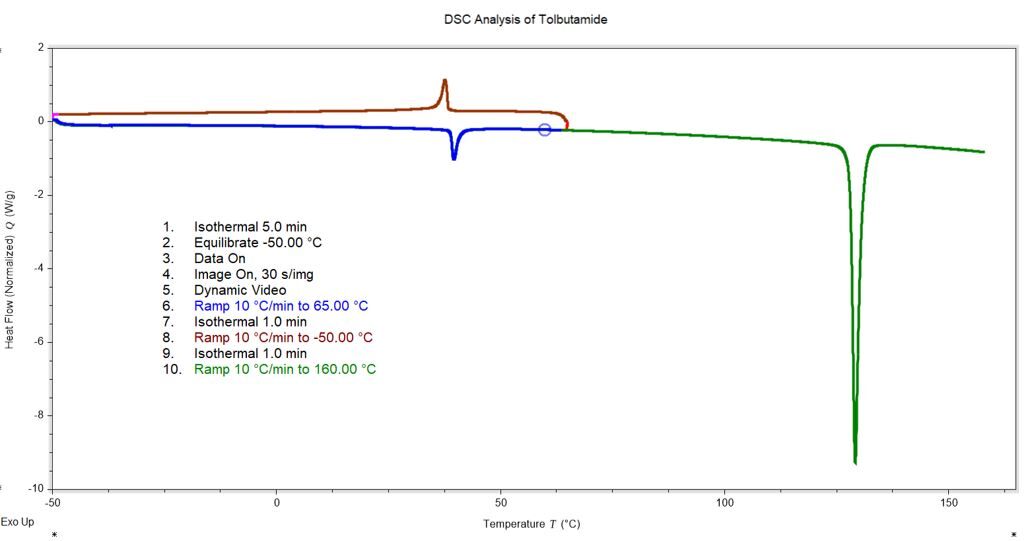
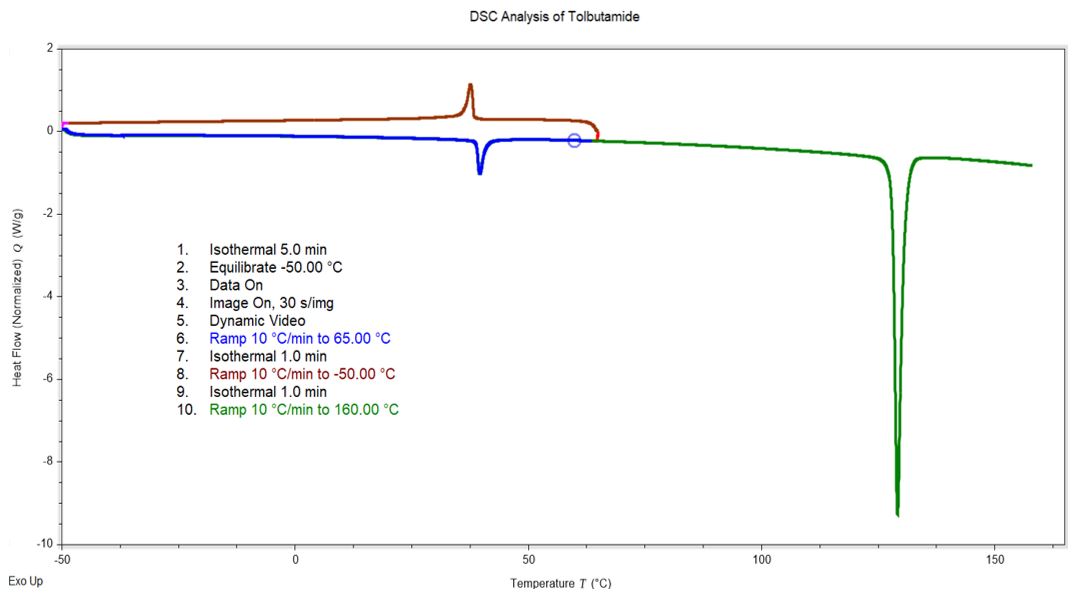
DSC Analysis of Tolbutamide
DSC Analysis of Tolbutamide
Tolbutamide Transition @ 40°C
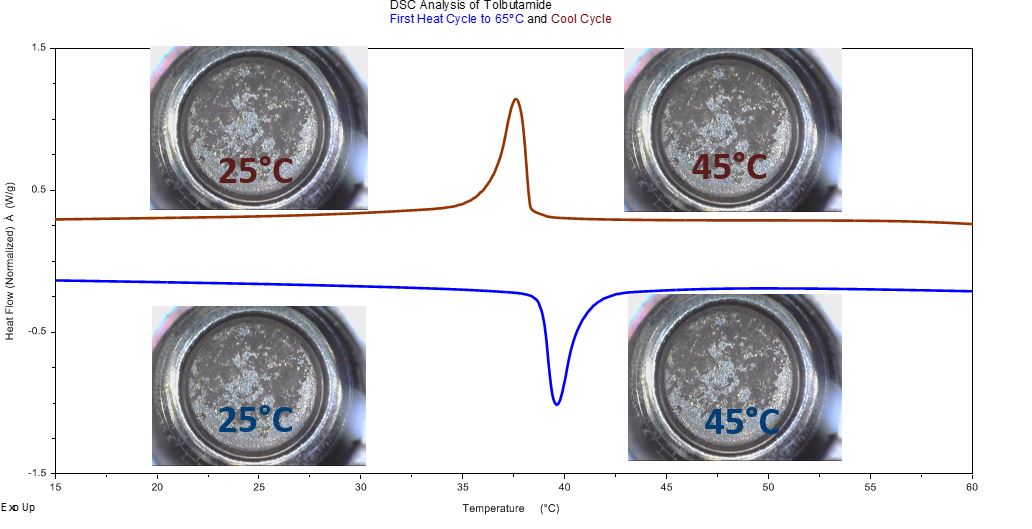
Tolbutamide transition @ 40°C is reversible and shows no change in physical appearance; typical for enantiotropic solid-solid phase conversion.
Tolbutamide: Melt Transition of As-Received Sample