Highest productivity, best accuracy, and widest humidity controlling range.


Accuracy for Best Sorption Data
A symmetric microbalance and advanced humidity chamber design deliver industry-leading stable baselines and weighing resolution. The SA provides accurate data for sorption analysis on small material quantities and/or on hydrophobic samples.

Productivity & Reliability
Autosampler and humidifier autofill pump allow for programming experimental queues of up to 10 or 25 samples. Even at high humidity and temperature or long lasting measurements, 24/7 productivity and absolute reliability are provided without user interaction.

Ease of use & Compliance
The App-style touchscreen and 21 CFR Part 11 compliant TRIOS software provide a unique user experience throughout the complete workflow from instrument control, method setup, and experiment to data evaluation.

Widest Humidity Control Range
Precise Wide-Range Humidity Control for Reliable Sorption Analysis The precise humidity control of the Discovery SA in combination with its industry leading weighing performance allows you to measure, analyze and optimize the water sorption properties of your sample material.
The precise humidity control of the Discovery SA in combination with its industry leading weighing performance allows you to measure, analyze and optimize the water sorption properties of your sample material.
Evaluating the sorption properties of advanced materials requires accurate control of the humidity from completely dry conditions to almost condensation. The Discovery SA provides humidity control from 0% RH to 98% RH in the entire temperature range from 5°C to 85°C. Only covering this whole range of humidity in small controllable increments allows assessment of the entire range of effects like surface adsorption, absorption, hydration or pore condensation.
A pair of mass flow controllers accurately meter and proportion gas to a symmetrical, well-insulated, aluminum block. The block contains a humidifier, gas transmission and mixing lines, plus easily accessible, identically arranged sample and reference measurement chambers. Temperature regulation of the block interior in the range from 5°C to 85°C is performed by thermoelectric (Peltier) devices and an accurate temperature sensor in a closed-loop system. The mass flow controllers adjust the amounts of wet (saturated) and dry gas to obtain humidities from 0% to 98% RH. Identical RH-sensors are located adjacent to the sample and reference crucibles and provide a continuous indication of humidity. Benefits of the design are the precise temperature control and a highly consistent atmosphere within the sample and reference chambers, which contribute to the excellent balance baseline stability and weighing sensitivity.
Humidity Control
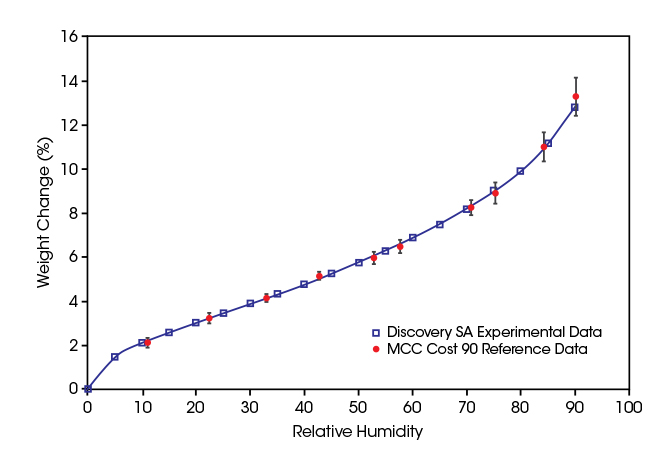
Microcrystalline cellulose (MCC) is a material with well characterized humidity sorption properties. In the plot below, humidity sorption data on MCC measured with the Discovery SA are plotted over RH. The red symbols in the plot are published, certified reference values from a COST 90 inter-laboratory test. The data measured with the Discovery SA are in agreement with the certified values within their confidence intervals over the whole RH range.
The excellent agreement found between the measured and reference data proves:
- The efficiency of the initial drying of the MCC at 0% RH
- The accuracy of the humidity and temperature control of the Discovery SA.
Humidity Control Verification
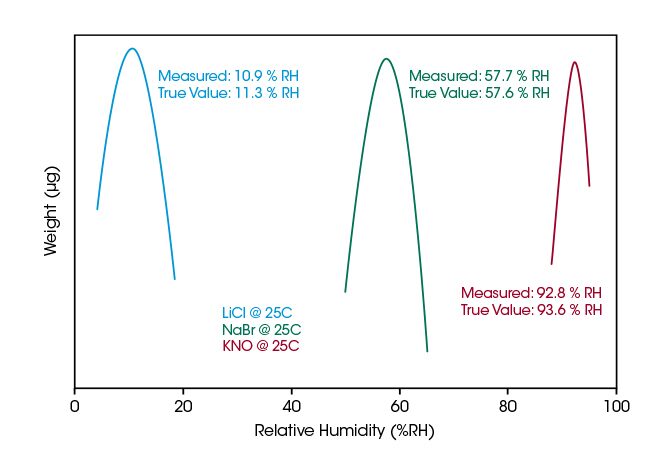
The Discovery SA TRIOS software has a built-in humidity verification feature and deliquescence method that allows the user to determine the humidity level at the sample. The method conforms to ASTM E2551.
The plot below summarizes humidity control verification data using three deliquescent salts at 25°C. The humidity control of the Discovery SA proves to be accurate within ±1% over the wide range from 11 to 93%.
Reliable Automation
The integral Discovery SA autosampler features a programmable multi-position sample carousel that permits automated analysis of up to 10 samples using semispherical quartz (or metalcoated quartz) crucibles and 25 samples using the optional tray with platinum or sealed aluminum pans. The design provides smooth and efficient loading and unloading of the sample pan without disturbing the balance. All aspects of sample testing are automated and software controlled including pan taring and loading, sample weighing, autosampler movement, humidity chamber movement and pan unloading. The productivity of the Discovery SA is maximized by the combination of the ingenious hardware with TRIOS software for pre-programmed analysis, automated data treatment, comparison, and presentation of results.


The Right Sample Pan for your Sample Material
Semispherical quartz, metal-coated quartz (180 μL) and optional platinum (100 μL) sample pans are available for use with the Discovery SA. The former are commonly used in sorption analysis because of their large volume, anti-static capabilities and open design, allowing good gas-sample contact and fast equilibration. Platinum pans are generic for TGA analysis of most materials, provide a good contact between sample and gas and can improve productivity with the 25-position autosampler tray. Sealed aluminum pans are available for ensuring the integrity of materials which readily adsorb moisture or lose volatiles. The sample is loaded into the aluminum pan which is then sealed with a lid before placing it in the autosampler tray. The isolated sample in the closed sample pan is not exposed to the environment. Immediately prior to loading the sample pan in the balance, the lid is automatically opened by the pan punch device in the autosampler.
Reliable Automation for Continuous Sorption Analysis without Monitoring 
Water in the humidifier is consumed during long sorption measurements or when performing measurements at high RH levels. In standard sorption analyzers, users are required to regularly check the water level and manually re-fill the humidifier. The new Discovery SA is the only instrument in the market which provides automatic humidifier filling. Level sensors measure the water level in the humidifier and control a refill pump. The refill pump automatically feeds water from an external storage bottle in the humidifier as required. Through this unique feature, the labor-intensive and error-prone manual monitoring of the water level is no longer necessary. Together with the autosampler, this feature increases the reliability and productivity of the sorption analyzer to a previously unprecedented level.
'App' Style touch Screen
 The app-style touch screen, powerful new TRIOS software and the robust, reliable autosampler with automated calibration and verification routines all work seamlessly to dramatically improve laboratory workflow and productivity.
The app-style touch screen, powerful new TRIOS software and the robust, reliable autosampler with automated calibration and verification routines all work seamlessly to dramatically improve laboratory workflow and productivity.
IT’S NEVER BEEN EASIER TO GET GREAT DATA!
Touch Screen Features and Benefits:
- Ergonomic design for easy viewing and operation
- Packed with functionality to simplify operation and enhance user experience. The touch screen includes:
- Start/stop runs
- Real-time plot
- Autosampler calibration
- Test and instrument status
- Active method viewing
- Loading/unloading and taring pans
- Real-time signals
- Advance methods segments
- System information
Measuring Practice
Sorption Analysis – Experimental Procedures
Sorption analysis quantifies the interaction of a sample material with humidity. For sorption analysis, the weight of the sample material is measured under controlled temperature (T) and relative humidity (RH) conditions. One of these properties – T or RH – is held constant while the other is changed either stepwise or continuously during a test. The table below provides an overview of the four flexible controlling modes which can be applied for performing sorption measurements with the Discovery SA.
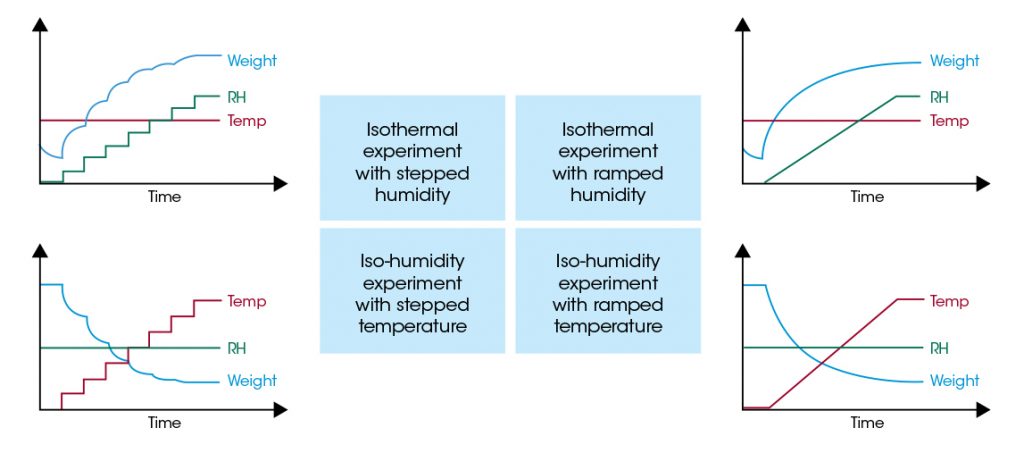
TRIOS software and the Discovery SA hardware design allow the user to choose the procedure which provides most useful data for the individual application case.
Stepwise changes of RH or T lead to an instantaneous change of the sample weight, which equilibrates to a new constant level after a sufficiently long time. The time needed for equilibration depends on the sample and the experimental conditions and characterizes the sorption kinetics of the material. Applying stepwise changes in RH provides the total amount of humidity sorption in addition to the kinetic of the uptake. This can be important information for determining diffusion coefficients of water into a sample material. For that reason, the stepwise change in RH at constant temperature is established as a quasi-standard method.
However, changing the temperature instead of the RH can also provide valuable information. Depending on the use case or the processing of a material, this process might mimic the application better than the RH change at a constant temperature. Temperature dependent sorption data allows for conclusions about the binding strength between the sample material and the sorbed water.
Continuously changing RH or T, however, leads to a continuous change in sample weight. If the sorption kinetics of the sample material is sufficiently fast, the resulting weight data are quasi-equilibrium sorption data measured in real time. The ramping procedures can provide contentious data sets in a comparably shorter time than the stepwise procedures provided that the kinetics of the sorption process is fast enough. This is another way to improve the productivity of the Discovery SA for sorption analysis.
Sorption Analysis – Isotherm and Isohume Plots
The weight of the sample material is recorded while T and RH are controlled. In the example below, a step change of RH or T initiates a weight change of the sample material. The microbalance continuously records the sample weight.
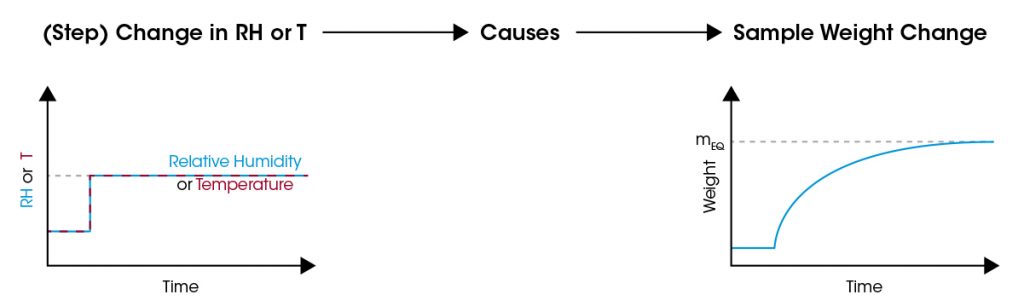
The recorded weight change rate over time is characteristic for the sorption kinetics. It indicates how fast humidity ad- or absorbs in the material or is released – desorbed – from the material. This is a characteristic property of the sample material. Weight changes can be fitted using TRIOS with an exponential model providing the time constant k of the sorption kinetics.
The sorption equilibrium is reached when the sample weight approaches a constant value (mEQ). The set of data at that time – RH, T, and mEQ – provides one point in the sorption isotherm or isohume. Data recorded in the same way at several RH or T values are used to plot a complete sorption isotherm or isohume as shown below.
Sorption Isotherm Plot (T = const.)
Isotherm plots indicate the influence of RH on water sorption. Isotherms are well suited to assess physical properties of the sample material and the type of sorption.
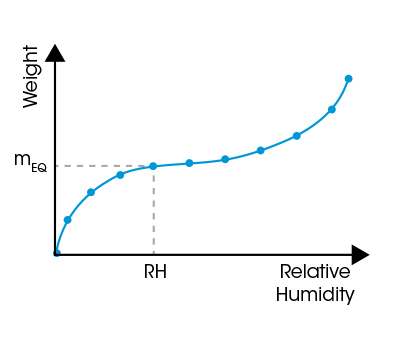
Sorption Isohume Plot (RH = const.)
Isothume plots illustrate the influence of T on water sorption. They are well suited to assess the chemical interaction between the material and water molecules.
Gas Blending
Gas Blending
Accurate Control of CO2 Concentration and Humidity for Assessment of Carbon Capture Materials

*maximum CO2 concentration is limited reverse proportional to RH: Max_CO2_conc. = 100% – RH
 Sorption Analysis of your Material with Ultimate Accuracy and Resolution
Sorption Analysis of your Material with Ultimate Accuracy and Resolution
At the core of every new Discovery SA is the proprietary Tru-Mass™ Balance. The Tru-Mass Balance system is actively temperature controlled for high sensitivity in every laboratory environment, delivers the highest resolution to accurately measure humidity sorption on the most challenging samples, and has ultra-low drift (Tru-Mass) for weight accuracy. Compared to competitive devices, the Discovery SA delivers higher weighing resolution and better baseline stability at all operating conditions. This guarantees industry leading accuracy for sorption analysis on small samples or for analyzing samples with a low sorpiton capacity.
Balance Features and Benefits:
- Ultra-low drift balance design ensures accurate detection of even the smallest weight changes
- High capacity (1 g) Tru-Mass balance with auto-ranging capability to ensure the best sensitivity no matter the size of the sample
- Temperature controlled balance with low drift and high sensitivity to deliver the most accurate
real-time data
The proprietary Tru-Mass™ balance delivers pure real-time weight data.
Industry Leading Weighing Performance
The Tru-Mass™ balance provides a weighing resolution of 0.01 μg over a 1000 mg weighing range. The sophisticated symmetrical balance design and efficient temperature control provide accurate weight measurements under all operating conditions.
The Discovery SA features isothermal baseline stability of ±0.25 μg over 24 h and low drift of ±1 μg over the temperature and humidity controlling range. With industry leading weighing performance capabilities, the Discovery SA can analyze of the most challenging samples with accuracy and ease.
| Test Condition | Baseline Stability |
|---|---|
| Isothermal at 25 °C and 20% RH over 24 h | ±0.25 μg |
| RH-ramping 5% to 85% | ±1 μg |
| T-ramping 25 °C to 85 °C | ±1 μg |

 TRIOS Software
TRIOS Software
Discover powerful TRIOS software that delivers exceptional user experience in a combined package for instrument control, data analysis, and reporting for thermal analysis and rheology. New features such as multiple calibration sets, real-time test method editing, and inter-laboratory data and test method sharing provide unmatched flexibility, while one-click analysis and custom reporting raise productivity to new levels.
TRIOS Features:
- Control multiple instruments with a single PC and software package
- Overlay and compare results across techniques including DSC, TGA, DMA, SDT
and rheometers - One-click repeated analysis for increased productivity
- Automated custom report generation including: experimental details, data plots and tables, and analysis results
- Convenient data export to plain-text, CSV, XML, Excel®, Word®, PowerPoint®,
and image formats - Optional TRIOS Guardian with electronic signatures for audit trail and data integrity
JSON Export
JSON Export: The Future of Data Management
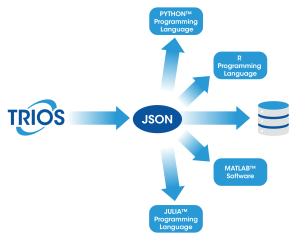
- Seamless Integration: Convert your TRIOS data into the open standard JSON format, making it easy to integrate with programming tools, data science workflows, and lab systems (e.g. LIMS). JSON is available:
- Automatically on every save (enabled in options)
- Through manual export dialogs
- As part of the “Send to LIMS” functionality
- Via the “Batch” processing dialog or from the command line
- In TRIOS AutoPilot
- Data Consistency: Our publicly available JSON schema ensures a consistent data structure, allowing you to write code once and apply it universally across all your data files.
- Python Library: Use our open-sourced python library, TA Data Kit, to simplify your data ingestion or learn how to take advantage of the power of our data with our code examples.
For more information, click here
Ease of Use
Ease of Use
TRIOS software makes calibration and operation simple. Users can easily generate multiple calibration or verification data sets under varying experimental conditions (e.g. different temperatures or humiditiy) and seamlessly switch between them to match the experimental conditions used for sample testing. Real-time signals and the progress of running experiments is readily available, with the added capability of modifying a running method on the fly. TRIOS software offers a level of flexibility that is unmatched in the industry.
Complete Data Record
The advanced data collection system automatically saves all relevant signals, active calibrations, and system settings. This comprehensive set of information is invaluable for method development, procedure deployment and data validation.
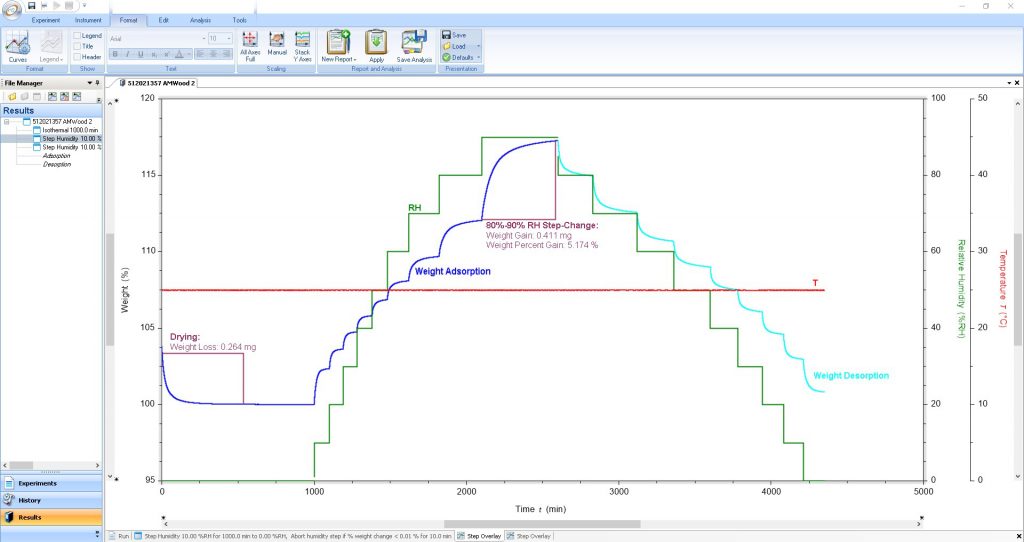
Complete Data Analysis Capabilities
Complete Data Analysis Capabilities
A comprehensive set of relevant tools are available for real-time data analysis, even during experiments. Gain actionable insights into your material’s behavior through a powerful and versatile set of features seamlessly integrated into TRIOS.
Standard SA Analyses:
- Plot sorption isotherm or isohume (weight change as function of relative humidity or temperature)
- Weight loss on drying
- Weight at specified time, relative humidity or temperature
- Onset and endset analysis
- Step transition analysis
- 1st and 2nd derivative
- Easily import and export SA data with TRIOS
Advanced Analysis Capabilities:
- Exponential and polynomial curve fit
- Sorption kinetics analysis
- Henry, Langmuir, DLP, BET, GAB sorption models
- Advanced custom analysis with user-defined variables and models
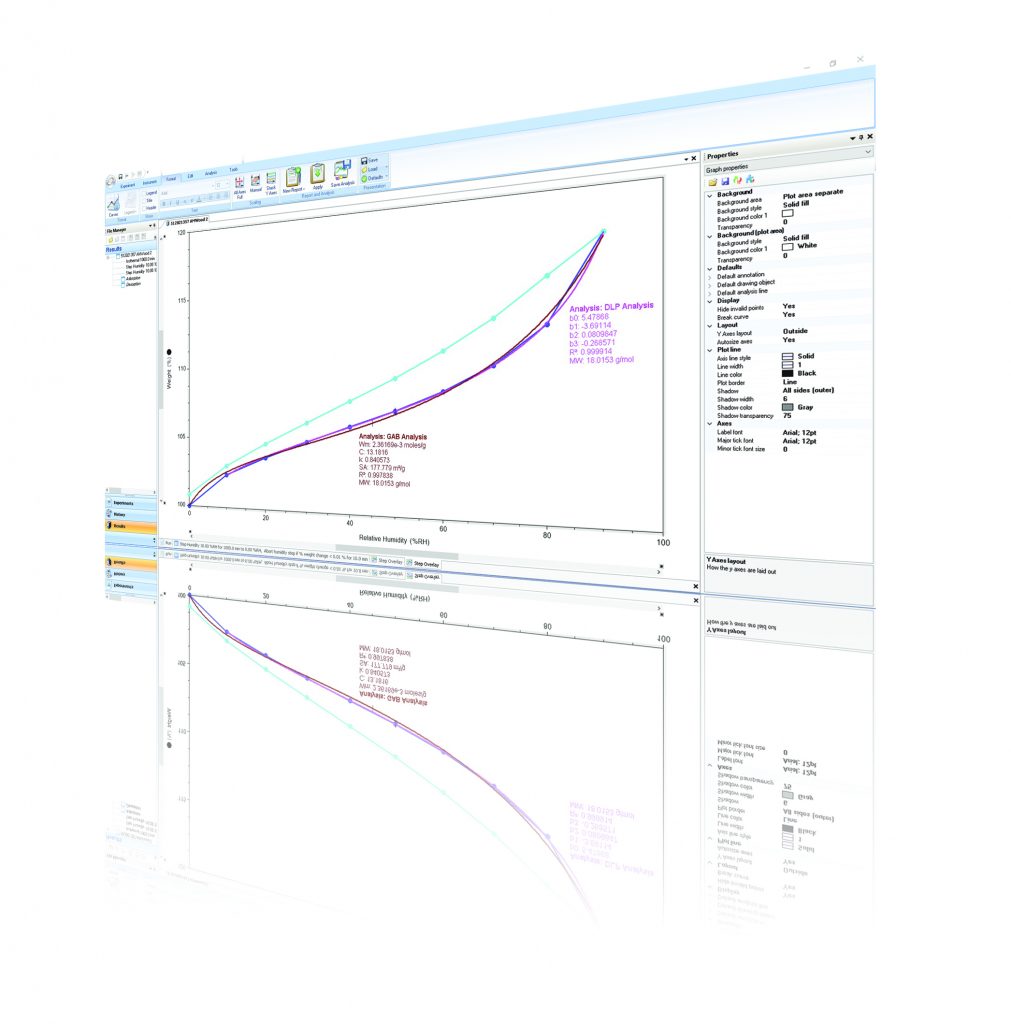 Sorption Isotherm Modeling
Sorption Isotherm Modeling
TRIOS is the only software covering the entire workflow from instrument control, data evaluation, modeling of the experimental data with a choice of different isotherm models and report generation. Experimental sorption isotherm data can be easily fitted with a choice of 5 sorption isotherm models. The parameters of the models determined through the data fit can be used to evaluate characteristic material properties, such as the specific surface area. The listing below provides a brief overview of the isotherm models in TRIOS and their characteristics.
Henry Isotherm
One-parameter model providing a linear relationship between RH and sorption. Typically used to describe only the linear part of the isotherm at low RH. The Henry parameter describes the slope of the isotherm in the origin.
Langmuir Isotherm
Two parameter model describing Type I isotherm shape with high uptake at low RH and a saturation at high RH. Parameters describe slope of the isotherm in the origin and the monolayer capacity allowing calculation of the specific surface area of the material.
BET Isotherm
Two parameter model describing Type II isotherms for multilayer sorption not approaching saturation. Parameters describe slope of the isotherm in the origin and the monolayer capacity allowing calculation of the specific surface area of the material.
GAB-Model
Three parameter modification of the BET isotherm extending the range of applicability to higher RH values. Parameters describe slope of the isotherm in the origin, the interaction of adsorbed molecules and the monolayer capacity allowing calculation of the specific surface area of the material.
DLP-Model
Four parameter polynomial model providing superior fitting flexibility for data interpolation. Parameters have no physical meaning.
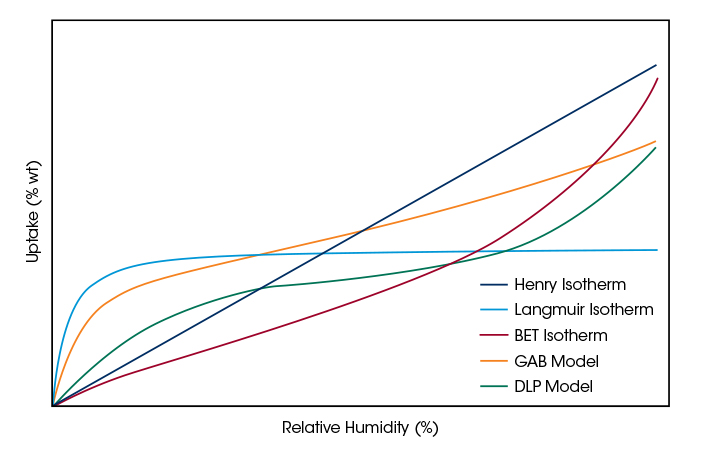
In a simple-to-apply procedure, TRIOS software determines the parameters of the models to obtain a best fit between the experimental sorption data and the physical or empirical model. The modeling allows users to interpolate between and extrapolate data points as well as to determine isosteric heats of adsorption. Parameters of the physical models provide insight about characteristic properties of the material, such as kinetics of adsorption and BET surface area.
Report Generation
Report Generation
Results can be exported as reports in graphic and numeric form. Predefined format templates can be applied for recurring report generation and help simplify the workflow.
TRIOS Guardian CFR Part 11 Compliance
TRIOS Guardian CFR Part 11 Compliance
Trios Guradian is a fully integrated solution providing CFR 21 Part 11 compliance. It uses the standard file system and does not require maintenance intensive and costly third-party database, hardware, or software. Guardian is designed primarily for laboratories in regulated environments and allows users to establish access limits and system recording protocols. These tools allow compliance with The Electronic Records and Electronic Signatures Rule (21 CFR Part 11) established by the Food and Drug Administration.
Features
- Restricted Access to Authorized Users: The System Administrator can restrict access to various features within the software, which defines the authorized user list. This list is coupled to work with all local and domain-based Windows user accounts.
- User Levels: Authorized users are assigned either a Standard User Level or a Basic User Level. Standard Users have access to the full software functionality.
- Audit Trail: Computer-generated time/date log of events (inherent to the software), including Windows User ID. Also includes the ability to claim authorship.
- Electronic Signatures: Users can electronically sign documents. The signature is added as an entry in the Results Log.
- Results Log: A record of the experimental test conditions and instrument parameters is saved within each data file. Any performed analysis and results functions are also stored within the file.
- PDF File Creation: TA Instruments’ software includes an embedded PDF file generator. This allows any printable document to be saved as a PDF file.
- File Check: Guardian automatically verifies that loaded data files have not been tampered with or modified. If the software detects any modification, the data file does not open and Guardian posts a message in the Notification Log. These events are also recorded in the Audit Trail.
Implementation
- Compatible with all Discovery Series Thermal Analysis instruments.
- Employs the standard TA Instruments software file system. No third-party database, hardware, or software is required.
- Interfaces directly into the PC Windows User Accounts and their associated enforced password policies.
Specifications
| Dynamic Weighing Range | – | 1000 mg |
| Weighing Resolution | – | 0.01 μg |
| Baseline Drift (Standard Deviation) | 24 h Isothermal 25° C and 20% RH | <±0.25 μg |
| RH-Ramp 5 %– 85% RH at 25° C | <±1 μg | |
| T-Ramp 25° C to 85° C at 20% RH | <±1 μg | |
| Sample Temperature | – | 5° C to 85 °C |
| Humidity Control Range | – | 0% to 98% RH |
| Humidity Accuracy | – | ±1% RH |
| Water Refill Pump | – | Standard Feature |
| Autosampler | 10 position | Standard Feature |
| 25 position | Optional, with platinum or sealed aluminum pans | |
| Sample Pans | Quartz or Metal-coated Quartz 180 μl | |
| Platinum 100 μl | ||
| Aluminum Sealed 20 μl | ||
Pharmaceuticals
Pharmaceuticals
Water or moisture is commonly found in pharmaceutical products. Water uptake by a drug substance is an inherent property. Raw materials or pharmaceutical products are exposed to water vapor during processing and storage. The efficacy and tolerability of drugs can be altered significantly by the effect of moisture on the active pharmaceutical ingredients and excipients. For this reason, the uptake capacity for moisture must be precisely known. The only way to protect the substances from undesirable changes caused by moisture is to limit the exposure to non-critical levels of humidity.
The United States Pharmacopeial Convention (USP) general chapter <1241> describes water-solid interactions as sorption. The degree of water sorption affects the crystallinity, permeability and melting point of pharmaceuticals. For amorphous materials, the presence of water can significantly alter the bulk properties like glass transition temperature, and may even initiate reversion to the crystalline form. Water also facilitates hydrolysis and induces drug degradation. Although it is not treated as an impurity, water in a drug substance is monitored and controlled as strictly as possible.
Assessing Hygroscopicity
The ability of materials to take up water vapor is often referred to as hygroscopicity. This material property is measured at a constant temperature with changing in RH while weighing the sample mass. These data provide an assessment of the potential effects of moisture on the properties of a pharmaceutical material and serve as criteria in selecting a drug for development. The following table classifies hygroscopicity of pharmaceutical substances as proposed by European Pharmacopeia.
Water sorption data are frequently used in the initial screening process to identify pharmaceutical candidate substances with low moisture uptake.
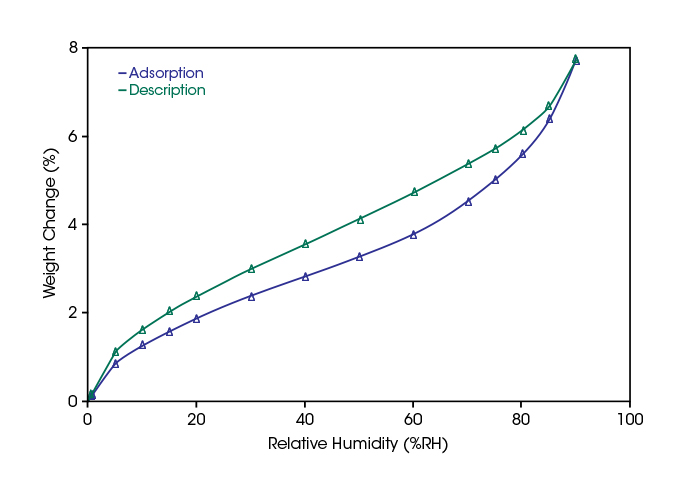
In the diagram below, the adsorption and desorption of water vapor on Ibuprofen at 25°C is shown as function of the relative humidity. According to the classification table, this substance would be considered moderately hygroscopic.
| Hygroscopicity Class | wt% Water Sorption at 25°C and 80% RH |
|---|---|
| Non Hygroscopic | 0 – 0.12 |
| Slightly Hygroscopic | 0.2 – 2.0 |
| Moderately Hygroscopic | 2.0 – 15.0 |
| Very Hygroscopic | >15.0 |
Assessing Amorphous – Crystalline Phase Changes
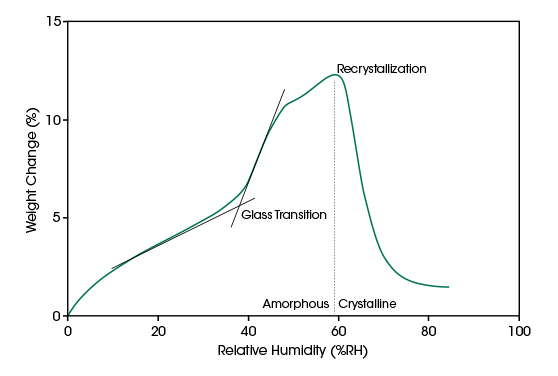
The extent of water vapor sorption depends on the structure of the material. The same material usually absorbs more water if it is in an amorphous state compared to the crystalline structure. Water sorption can lower the glass transition temperature significantly and initiate recrystallization.
An isothermal experiment with ramped RH is useful to identify vapor sorption induced phase transitions. The non-linear moisture absorption of the material indicates the glass transition. Recrystallization leads to a desorption of water with increasing RH. In the diagram below, the weight change of an amorphous lactose sample recorded with ramped RH at 25°C is plotted.
Assessing Hydrate Formation
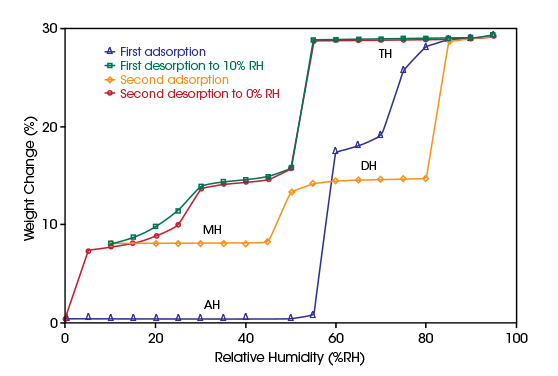
Approximately one-third of all active pharmaceutical substances (APIs) form hydrates. Spontaneous hydration by moisture in the air can occur at any stage of drug production or storage leading to hydrate formation. The state of hydration changes several properties including physical and chemical stability. Hydrated materials can become amorphous during dehydration, and hydrates affect material solubility, dissolution and bioavailability. Throughout the entire workflow from preformulation to the manufacturing process, packaging and storage, the physical form of excipients and APIs must be fully characterized and controlled. Water vapor sorption is the ideal tool to detect and characterize hydrate formation as a function of temperature and relative humidity.Water vapor adsorption and desorption of anhydrous (AH) sodium naproxen as function of changing RH was investigated in the Discovery SA at 25°C. The stepwise weight changes of the material plotted in the top diagram indicate the formation of monohydrate (MH), dihydrate (DH), and the tetrahydrate (TH).
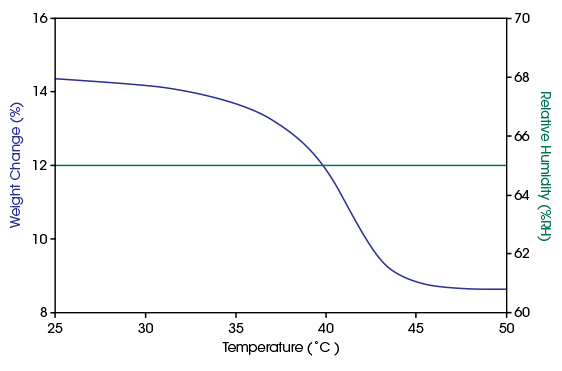
In the bottom diagram, the results of an isohume measurement at 65%RH with ramping the temperature from 25°C to 50°C are shown. At 25°C the material is in dihydrated condition. With increasing temperature, it dehydrates into a monohydrate which is completed at temperatures higher than 45°C.
Polymers
Polymeric materials are applied widely in manufacturing of consumer products and as packaging material. Many polymers have a natural tendency to absorb water from their humid environment. Absorbed water has been shown to act as a plasticizer, reducing the glass transition temperature and mechanical strength. However, absorbed water also can lead to irreversible degradation of the polymer structure.
Gravimetric vapor sorption measurements are suggested in ASTM, ISO and other technical standards for evaluating polymer water interactions. The Discovery SA measures the water absorption as weight increase of a polymer material exposed to a controlled RH allowing to assess the hygroscopic stability of the material. The kinetics of water uptake or release is characteristic for the water permeability of the polymer material and can be extracted from the continuously recorded weight data.
Hydrolysis Stability of Polymers for Electronic Devices
In electronic device manufacturing, reliability issues associated with water absorption have become increasingly important. Advanced polymer based materials are applied for more function integration and further miniaturization. Their properties must remain intact when they are exposed to environmental humidity.
Kapton is a polyimide polymer that remains stable in dry conditions in a wide temperature range. Kapton is used as base material for printed circuits for flexible electronics and as an insulation and protection layer on electrostatic sensitive and fragile components. The improved electrical, chemical, and mechanical properties of Kapton compared to other commonly used polyimide materials are a result of its significantly improved hydrolysis resistance.
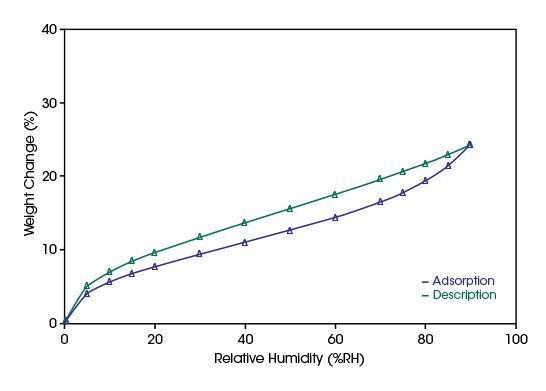
In the diagram below, water vapor ab- and desorption data measured on a Kapton tape at 25°C are plotted. As expected, the water vapor sorption is found to be small compared to other polyimide polymers.
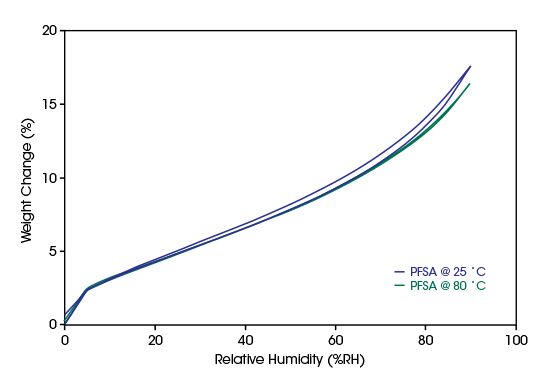
Assessing Water Sorption of Fuel-Cell Membranes
Improvements in electrochemical conversion of water have been achieved through the development of new proton exchange membrane materials (PEM). Conversion of hydrogen and oxygen into water in fuel cells relies on a PEM. The same is true for the conversion of water to hydrogen and oxygen in electrolysers. In both, the PEMs form the heart of the electrochemical cells and understanding the mechanisms of their degradation allows researchers to develop more reliable and effective fuel cells and electrolysers.In the plot below, the water vapor ab- and desorption on a perfluorosulfonic acid membrane is compared for 25°C and 80°C. While the amount of water sorption is almost unchanged, the hysteresis between ad- and desorption disappears at the higher temperature. This indicates a higher reversibility of water sorption at higher temperatures improving the removal of the reaction product from the membrane material.
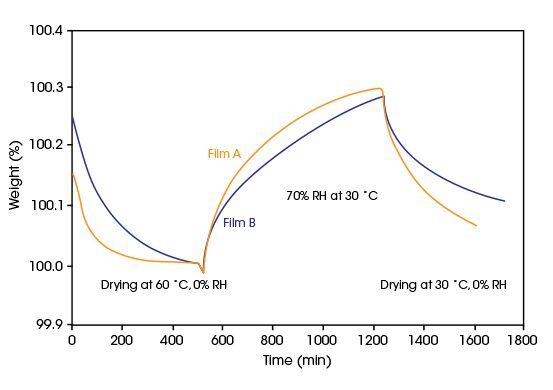
Water Permeation in Packaging Polymer Films
The first step of water permeation through a polymeric packaging material is the absorption of moisture from the environment. A low water vapor absorption capacity and/or a slow kinetics of ab- and desorption indicate a low permeability. Water vapor sorption is an extremely valuable tool for comparing polymeric films used for packaging drugs and other humidity sensitive products.The plot below compares sorption kinetics for two different polymeric packaging films undergoing temperature and relative humidity cycling. Film A absorbs and desorbs moisture at a more rapid rate than the other film. Higher sorption capacity and faster sorption kinetics suggest that film A is less suitable for packaging moisture sensitive materials than film B.
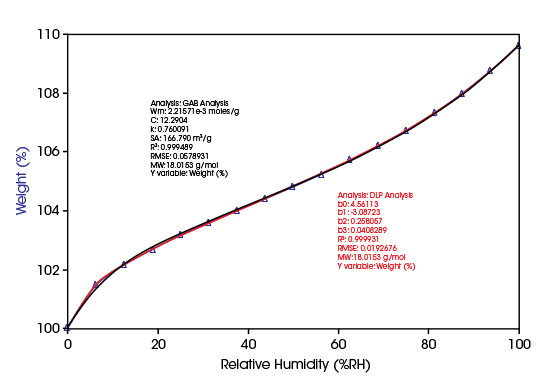
Assessing Hydroscopicity of Natural Polymers
Microcrystalline cellulose (MCC) is a naturally occurring polymer. MCC is a valuable additive in pharmaceutical, food, cosmetic and other industries. Amongst other properties of MCC, moisture sorption capacity and moisture content are measured to qualify its suitability to such utilization.
In the diagram, the moisture sorption isotherm data of MCC are shown together with a fit of the data with the GAB and the DLP models. While the parameters of the DLP model have no physical meaning, the GAB parameter characterizing the monolayer sorption capacity Wm = 2.2×10-3 mol/g allows the calculation of the specific surface area of the material:
SA = Wm×N×AW with N = 6.0221×1023 molecules/mol and AW = 12.5×10-20 m2/molecule
SA,MCC = 166 m2/g
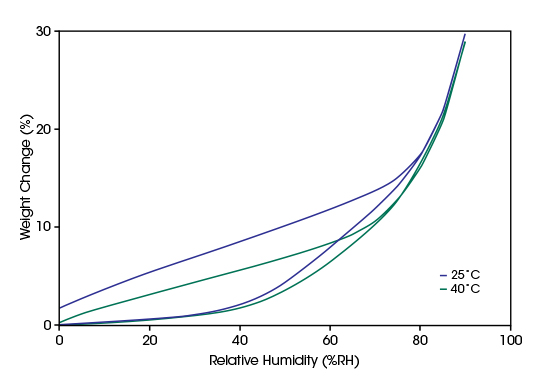
Food
Food
Moisture content is a critical factor to consider in the food industry. The amount of water in a product affects the product’s texture, shelf life, ease of processing, and cost to produce. Increased moisture content of food can make crispy food products soft or fresh pasta sticky and difficult to manage. On the other hand, if the product is too dry, lack of moisture can make it brittle, or hard as a rock. Further, microbial activity increases with the moisture availability in the food. Moisture rich foods are easily susceptible to microbial attacks, rot, and damage. Thus, the shelf life of the food material is determined by the moisture content in the food.
Developing suitable recipes and optimal processing and storage conditions allows manufacturers to control the moisture absorption of food from the atmosphere. Well conditioned foods with controlled moisture sorption preserve the taste and desired texture, provide a better shelf life and enhance the customer experience.
Assessing shelf Life and Storage Stability
Crispness is one of the most important sensory quality attributes of corn flakes. It should be preserved when the corn flakes are stored after the pack is opened. This requires low moisture sorption at lower humidity levels. At high humidity, the moisture sorption should increase considerably to allow the milk to penetrate the flake before consumption.
In the diagram below, water vapor ab- and desorption data measured on corn flakes at 25°C and 40°C are compared. The sorption isotherms exhibit at both temperatures the desired type III shape with low moisture sorption up to 40%RH. This indicates that in the investigated range, temperature has no significant influence on the storage stability of corn flakes.
Assessing Hydroscopicity of Corn Starch
Starch is one of the most important bio-polymer components of cereals, which determines to a great extent their hygroscopic properties. Starch is also used in many food products, whose preservation depends essentially on its humidity sorption properties. Owing to its versatile properties and variability, starch is also used in the production of packaging materials, biotechnology, fragrance, textile, and pharmaceutical products.
In the diagram below, the ab- and desorption of water vapor in corn starch measured at 25°C is plotted as function of RH. The continuous progressing sorption isotherm type II and the relatively small hysteresis are characteristic for corn starch.
Construction Materials
Construction Materials
The moisture absorption properties of building materials are critical to improving durability, designing low-energy building structures, and effective impregnation. Ultimately, materials with controlled moisture sorption properties are critical for resident comfort and well-being.
Humidity or moisture is considered one of the highly relevant factors to the reliability and proper functioning of building structures. In particular, for building materials moisture sorption has significant implications for stones, cements, woods, and insulation materials. Moisture damage is a significant factor limiting a building’s lifespan. As well, moisture infusion through a building’s outer structure can have a significant effect on indoor air quality and air-conditioning load.
The water vapor sorption isotherm is one of the main parameters necessary for the analysis of material’s hygroscopicity and moisture transport between the building environment and the indoor air.
Assessing Water Sorption in Wood
Wood is a key natural resource and a versatile material used in building and construction applications. Its structural properties vary with the water content and it is subject to natural decay processes. Therefore, it is necessary to understand the water sorption in wood as a function of humidity.
Protection of wood can be achieved by preventing water transport across the material, through sealing the surfaces and treating the wood so that it does not absorb moisture. The analysis of the susceptibility of wood to natural decay and the suitability of wood for construction can be performed through vapor sorption measurements.
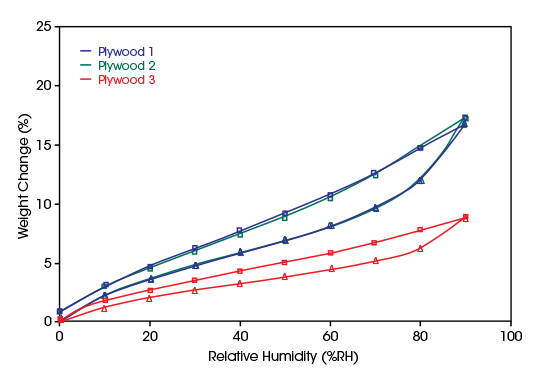
In the diagram, the ad- and desorption of water vapor on three plywood samples of different densities are compared.
Adsorbents and Catalysts
Adsorbents and Catalysts
 Developing water tolerant adsorbent materials and adsorption processes is essential for cost and energy efficient purification and gas storage processes. Measuring water vapor adsorption isotherms on the materials is key information for improving their properties. Adsorbent materials are used in a wide range of industrial and environmental applications, including purification and separation of mixtures, drying, catalysis, pollution control and others. Most materials have a porous texture with a high specific surface area. In many separation applications – except in drying – water is not considered a pollutant which should be adsorbed. Adsorbed water blocks adsorption capacity and reduces the efficiency of the material. Some adsorbents, such as novel metal-organic networks (MOF), which excel in gas storage and purification due to their very high porosity, are not stable in the presence of water.
Developing water tolerant adsorbent materials and adsorption processes is essential for cost and energy efficient purification and gas storage processes. Measuring water vapor adsorption isotherms on the materials is key information for improving their properties. Adsorbent materials are used in a wide range of industrial and environmental applications, including purification and separation of mixtures, drying, catalysis, pollution control and others. Most materials have a porous texture with a high specific surface area. In many separation applications – except in drying – water is not considered a pollutant which should be adsorbed. Adsorbed water blocks adsorption capacity and reduces the efficiency of the material. Some adsorbents, such as novel metal-organic networks (MOF), which excel in gas storage and purification due to their very high porosity, are not stable in the presence of water.
Water Adsorption on Hydrophilic Absorbents
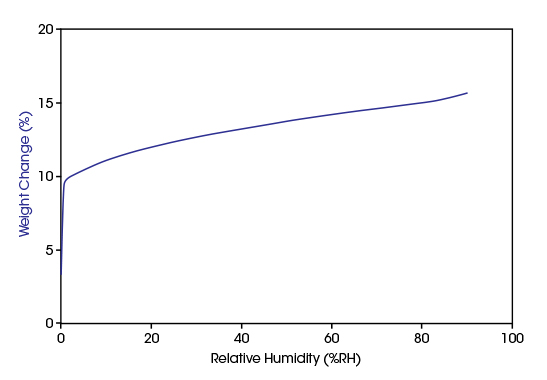
Zeolites are microporous, aluminosilicate minerals bearing a negatively charged honeycomb framework of micropores into which molecules may be adsorbed. Zeolites occur naturally but are also produced industrially on a large scale. A-type zeolites are used industrially in natural gas drying and desulfurization as well as separation of nitrogen and oxygen.
Due to their polar nature, instantaneous water adsorption happens at a low RH level. This behavior can be observed as the typical steep type I isotherm in the diagram. The Discovery SA can control RH in small increments, so that the steep increasing branch of the isotherm can be analyzed.
Water Adsorption on Hydrophobic Absorbents
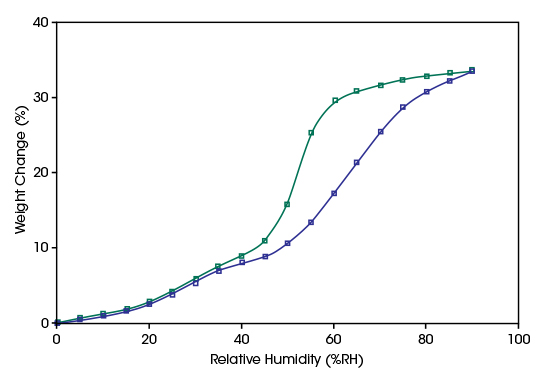
Activated carbons are the most widely used industrial adsorbent for removing contaminants and pollutants from gaseous, aqueous, and nonaqueous streams. They are inexpensive to manufacture and have uniquely powerful adsorption properties. Depending on the starting material and the activation process, a wide range of pore structures can be generated which makes porous carbons applicable to a wide range of technologies. Their non-polar interface leads to weak interactions with water vapor. As a result, the isotherm is of type III with a low uptake at low RH levels. The increasing water sorption at higher RH levels is due to pore condensation. Between the adsorption and desorption branch of the isotherm, a hysteresis is formed which is characteristic for the pore size distribution of the activated carbon.
CO2 Capture
CO2 Capture
Gas blending and humidity control providing flexibility to assess carbon capture materials
Humidity sorption at controlled CO2 levels
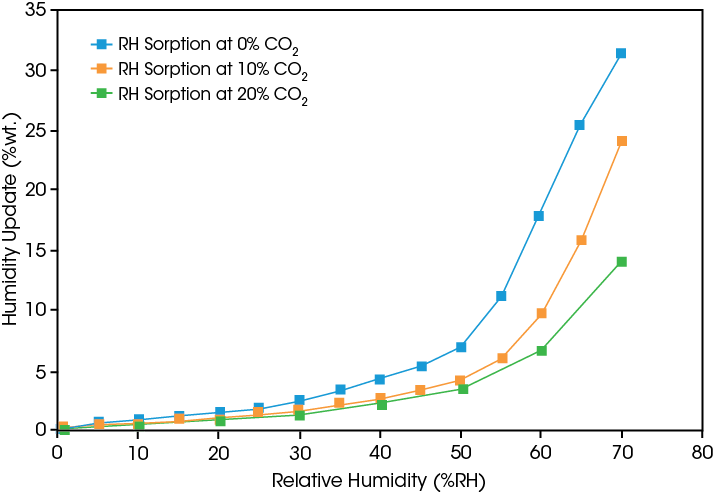
CO2 sorption at controlled RH
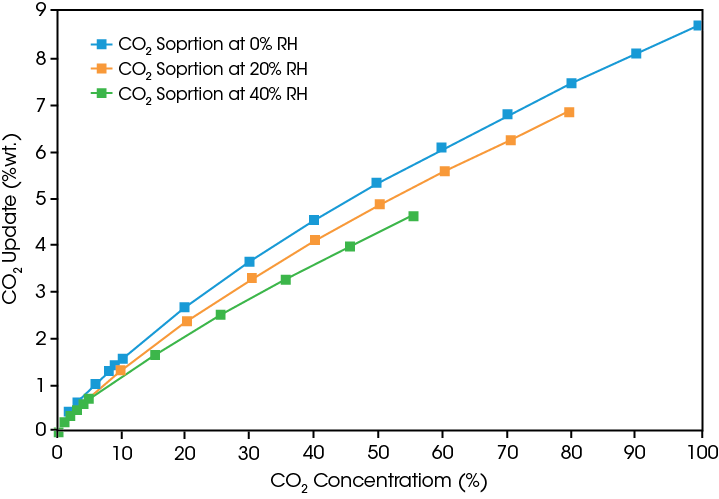
- Description
-
TA Instruments invites you to experience the world’s most productive Dynamic Vapor Sorption (DVS) Analyzer, the Discovery SA. Discover the advanced engineering and attention to detail that provides enhancements in every aspect of DVS technology and a new level of user experience. With industry-leading performance, exceptionally wide humidity control range and ease of use, the Discovery SA meets your needs and exceeds your expectations.

Accuracy for Best Sorption Data
A symmetric microbalance and advanced humidity chamber design deliver industry-leading stable baselines and weighing resolution. The SA provides accurate data for sorption analysis on small material quantities and/or on hydrophobic samples.

Productivity & Reliability
Autosampler and humidifier autofill pump allow for programming experimental queues of up to 10 or 25 samples. Even at high humidity and temperature or long lasting measurements, 24/7 productivity and absolute reliability are provided without user interaction.

Ease of use & Compliance
The App-style touchscreen and 21 CFR Part 11 compliant TRIOS software provide a unique user experience throughout the complete workflow from instrument control, method setup, and experiment to data evaluation.

- Technology
-
Widest Humidity Control Range
Precise Wide-Range Humidity Control for Reliable Sorption Analysis
 The precise humidity control of the Discovery SA in combination with its industry leading weighing performance allows you to measure, analyze and optimize the water sorption properties of your sample material.
The precise humidity control of the Discovery SA in combination with its industry leading weighing performance allows you to measure, analyze and optimize the water sorption properties of your sample material.Evaluating the sorption properties of advanced materials requires accurate control of the humidity from completely dry conditions to almost condensation. The Discovery SA provides humidity control from 0% RH to 98% RH in the entire temperature range from 5°C to 85°C. Only covering this whole range of humidity in small controllable increments allows assessment of the entire range of effects like surface adsorption, absorption, hydration or pore condensation.
A pair of mass flow controllers accurately meter and proportion gas to a symmetrical, well-insulated, aluminum block. The block contains a humidifier, gas transmission and mixing lines, plus easily accessible, identically arranged sample and reference measurement chambers. Temperature regulation of the block interior in the range from 5°C to 85°C is performed by thermoelectric (Peltier) devices and an accurate temperature sensor in a closed-loop system. The mass flow controllers adjust the amounts of wet (saturated) and dry gas to obtain humidities from 0% to 98% RH. Identical RH-sensors are located adjacent to the sample and reference crucibles and provide a continuous indication of humidity. Benefits of the design are the precise temperature control and a highly consistent atmosphere within the sample and reference chambers, which contribute to the excellent balance baseline stability and weighing sensitivity.
Humidity Control
Microcrystalline cellulose (MCC) is a material with well characterized humidity sorption properties. In the plot below, humidity sorption data on MCC measured with the Discovery SA are plotted over RH. The red symbols in the plot are published, certified reference values from a COST 90 inter-laboratory test. The data measured with the Discovery SA are in agreement with the certified values within their confidence intervals over the whole RH range.
The excellent agreement found between the measured and reference data proves:
- The efficiency of the initial drying of the MCC at 0% RH
- The accuracy of the humidity and temperature control of the Discovery SA.

Humidity Control Verification

The Discovery SA TRIOS software has a built-in humidity verification feature and deliquescence method that allows the user to determine the humidity level at the sample. The method conforms to ASTM E2551.
The plot below summarizes humidity control verification data using three deliquescent salts at 25°C. The humidity control of the Discovery SA proves to be accurate within ±1% over the wide range from 11 to 93%.

Reliable Automation
Improved Productivity in Sorption AnalysisThe integral Discovery SA autosampler features a programmable multi-position sample carousel that permits automated analysis of up to 10 samples using semispherical quartz (or metalcoated quartz) crucibles and 25 samples using the optional tray with platinum or sealed aluminum pans. The design provides smooth and efficient loading and unloading of the sample pan without disturbing the balance. All aspects of sample testing are automated and software controlled including pan taring and loading, sample weighing, autosampler movement, humidity chamber movement and pan unloading. The productivity of the Discovery SA is maximized by the combination of the ingenious hardware with TRIOS software for pre-programmed analysis, automated data treatment, comparison, and presentation of results.


The Right Sample Pan for your Sample Material

Semispherical quartz, metal-coated quartz (180 μL) and optional platinum (100 μL) sample pans are available for use with the Discovery SA. The former are commonly used in sorption analysis because of their large volume, anti-static capabilities and open design, allowing good gas-sample contact and fast equilibration. Platinum pans are generic for TGA analysis of most materials, provide a good contact between sample and gas and can improve productivity with the 25-position autosampler tray. Sealed aluminum pans are available for ensuring the integrity of materials which readily adsorb moisture or lose volatiles. The sample is loaded into the aluminum pan which is then sealed with a lid before placing it in the autosampler tray. The isolated sample in the closed sample pan is not exposed to the environment. Immediately prior to loading the sample pan in the balance, the lid is automatically opened by the pan punch device in the autosampler.
Reliable Automation for Continuous Sorption Analysis without Monitoring

Water in the humidifier is consumed during long sorption measurements or when performing measurements at high RH levels. In standard sorption analyzers, users are required to regularly check the water level and manually re-fill the humidifier. The new Discovery SA is the only instrument in the market which provides automatic humidifier filling. Level sensors measure the water level in the humidifier and control a refill pump. The refill pump automatically feeds water from an external storage bottle in the humidifier as required. Through this unique feature, the labor-intensive and error-prone manual monitoring of the water level is no longer necessary. Together with the autosampler, this feature increases the reliability and productivity of the sorption analyzer to a previously unprecedented level.
'App' Style touch Screen
 The app-style touch screen, powerful new TRIOS software and the robust, reliable autosampler with automated calibration and verification routines all work seamlessly to dramatically improve laboratory workflow and productivity.
The app-style touch screen, powerful new TRIOS software and the robust, reliable autosampler with automated calibration and verification routines all work seamlessly to dramatically improve laboratory workflow and productivity.IT’S NEVER BEEN EASIER TO GET GREAT DATA!
Touch Screen Features and Benefits:

- Ergonomic design for easy viewing and operation
- Packed with functionality to simplify operation and enhance user experience. The touch screen includes:
- Start/stop runs
- Real-time plot
- Autosampler calibration
- Test and instrument status
- Active method viewing
- Loading/unloading and taring pans
- Real-time signals
- Advance methods segments
- System information
Measuring Practice
Sorption Analysis – Experimental Procedures
Sorption analysis quantifies the interaction of a sample material with humidity. For sorption analysis, the weight of the sample material is measured under controlled temperature (T) and relative humidity (RH) conditions. One of these properties – T or RH – is held constant while the other is changed either stepwise or continuously during a test. The table below provides an overview of the four flexible controlling modes which can be applied for performing sorption measurements with the Discovery SA.

TRIOS software and the Discovery SA hardware design allow the user to choose the procedure which provides most useful data for the individual application case.
Stepwise changes of RH or T lead to an instantaneous change of the sample weight, which equilibrates to a new constant level after a sufficiently long time. The time needed for equilibration depends on the sample and the experimental conditions and characterizes the sorption kinetics of the material. Applying stepwise changes in RH provides the total amount of humidity sorption in addition to the kinetic of the uptake. This can be important information for determining diffusion coefficients of water into a sample material. For that reason, the stepwise change in RH at constant temperature is established as a quasi-standard method.
However, changing the temperature instead of the RH can also provide valuable information. Depending on the use case or the processing of a material, this process might mimic the application better than the RH change at a constant temperature. Temperature dependent sorption data allows for conclusions about the binding strength between the sample material and the sorbed water.
Continuously changing RH or T, however, leads to a continuous change in sample weight. If the sorption kinetics of the sample material is sufficiently fast, the resulting weight data are quasi-equilibrium sorption data measured in real time. The ramping procedures can provide contentious data sets in a comparably shorter time than the stepwise procedures provided that the kinetics of the sorption process is fast enough. This is another way to improve the productivity of the Discovery SA for sorption analysis.
Sorption Analysis – Isotherm and Isohume Plots
The weight of the sample material is recorded while T and RH are controlled. In the example below, a step change of RH or T initiates a weight change of the sample material. The microbalance continuously records the sample weight.

The recorded weight change rate over time is characteristic for the sorption kinetics. It indicates how fast humidity ad- or absorbs in the material or is released – desorbed – from the material. This is a characteristic property of the sample material. Weight changes can be fitted using TRIOS with an exponential model providing the time constant k of the sorption kinetics.
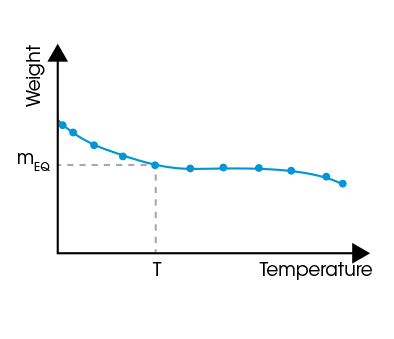
The sorption equilibrium is reached when the sample weight approaches a constant value (mEQ). The set of data at that time – RH, T, and mEQ – provides one point in the sorption isotherm or isohume. Data recorded in the same way at several RH or T values are used to plot a complete sorption isotherm or isohume as shown below.
Sorption Isotherm Plot (T = const.)
Isotherm plots indicate the influence of RH on water sorption. Isotherms are well suited to assess physical properties of the sample material and the type of sorption.

Sorption Isohume Plot (RH = const.)
Isothume plots illustrate the influence of T on water sorption. They are well suited to assess the chemical interaction between the material and water molecules.

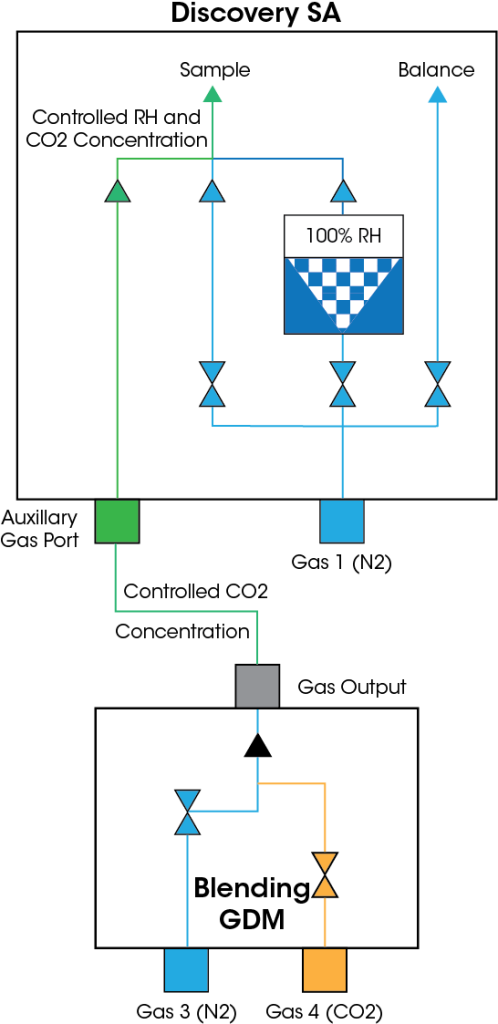
Gas Blending
Gas Blending
Accurate Control of CO2 Concentration and Humidity for Assessment of Carbon Capture Materials
A Blending Gas Dosing Module (Blending GDM) can be used with the Discovery SA allowing for automated blending of CO2 into the humid carrier gas flow. The Blending GDM is an external accessory with two gas inlet ports that, when connected to the auxiliary gas port on the SA, gives the user a total of two gases (CO2 and N2) and humidity to control. The added blending capability allows for SA experiments to be carried out in a humidity-controlled atmosphere where the concentration* of CO2 may be fixed, stepped incrementally or ramped at a controlled rate.
*maximum CO2 concentration is limited reverse proportional to RH: Max_CO2_conc. = 100% – RH
- Performance
-
 Sorption Analysis of your Material with Ultimate Accuracy and Resolution
Sorption Analysis of your Material with Ultimate Accuracy and ResolutionAt the core of every new Discovery SA is the proprietary Tru-Mass™ Balance. The Tru-Mass Balance system is actively temperature controlled for high sensitivity in every laboratory environment, delivers the highest resolution to accurately measure humidity sorption on the most challenging samples, and has ultra-low drift (Tru-Mass) for weight accuracy. Compared to competitive devices, the Discovery SA delivers higher weighing resolution and better baseline stability at all operating conditions. This guarantees industry leading accuracy for sorption analysis on small samples or for analyzing samples with a low sorpiton capacity.
Balance Features and Benefits:
- Ultra-low drift balance design ensures accurate detection of even the smallest weight changes
- High capacity (1 g) Tru-Mass balance with auto-ranging capability to ensure the best sensitivity no matter the size of the sample
- Temperature controlled balance with low drift and high sensitivity to deliver the most accurate
real-time data
The proprietary Tru-Mass™ balance delivers pure real-time weight data.
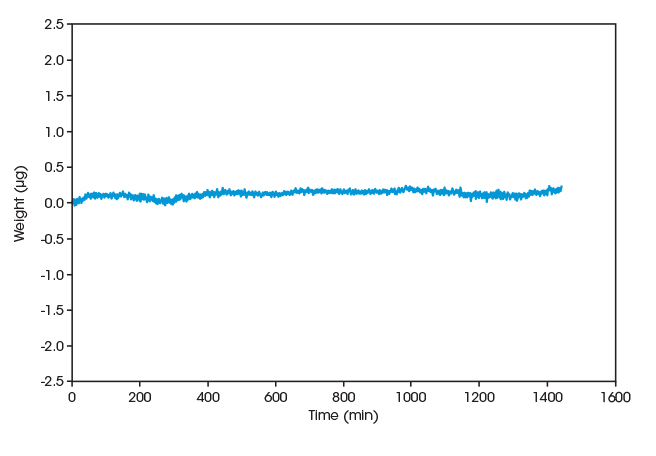
Industry Leading Weighing Performance
The Tru-Mass™ balance provides a weighing resolution of 0.01 μg over a 1000 mg weighing range. The sophisticated symmetrical balance design and efficient temperature control provide accurate weight measurements under all operating conditions.
The Discovery SA features isothermal baseline stability of ±0.25 μg over 24 h and low drift of ±1 μg over the temperature and humidity controlling range. With industry leading weighing performance capabilities, the Discovery SA can analyze of the most challenging samples with accuracy and ease.
Test Condition Baseline Stability Isothermal at 25 °C and 20% RH over 24 h ±0.25 μg RH-ramping 5% to 85% ±1 μg T-ramping 25 °C to 85 °C ±1 μg 

- Software
-
 TRIOS Software
TRIOS SoftwareDiscover powerful TRIOS software that delivers exceptional user experience in a combined package for instrument control, data analysis, and reporting for thermal analysis and rheology. New features such as multiple calibration sets, real-time test method editing, and inter-laboratory data and test method sharing provide unmatched flexibility, while one-click analysis and custom reporting raise productivity to new levels.
TRIOS Features:
- Control multiple instruments with a single PC and software package
- Overlay and compare results across techniques including DSC, TGA, DMA, SDT
and rheometers - One-click repeated analysis for increased productivity
- Automated custom report generation including: experimental details, data plots and tables, and analysis results
- Convenient data export to plain-text, CSV, XML, Excel®, Word®, PowerPoint®,
and image formats - Optional TRIOS Guardian with electronic signatures for audit trail and data integrity
JSON Export
JSON Export: The Future of Data Management

- Seamless Integration: Convert your TRIOS data into the open standard JSON format, making it easy to integrate with programming tools, data science workflows, and lab systems (e.g. LIMS). JSON is available:
- Automatically on every save (enabled in options)
- Through manual export dialogs
- As part of the “Send to LIMS” functionality
- Via the “Batch” processing dialog or from the command line
- In TRIOS AutoPilot
- Data Consistency: Our publicly available JSON schema ensures a consistent data structure, allowing you to write code once and apply it universally across all your data files.
- Python Library: Use our open-sourced python library, TA Data Kit, to simplify your data ingestion or learn how to take advantage of the power of our data with our code examples.
For more information, click here
Ease of Use
Ease of Use
TRIOS software makes calibration and operation simple. Users can easily generate multiple calibration or verification data sets under varying experimental conditions (e.g. different temperatures or humiditiy) and seamlessly switch between them to match the experimental conditions used for sample testing. Real-time signals and the progress of running experiments is readily available, with the added capability of modifying a running method on the fly. TRIOS software offers a level of flexibility that is unmatched in the industry.

Complete Data Record
Complete Data RecordThe advanced data collection system automatically saves all relevant signals, active calibrations, and system settings. This comprehensive set of information is invaluable for method development, procedure deployment and data validation.

Complete Data Analysis Capabilities
Complete Data Analysis Capabilities
A comprehensive set of relevant tools are available for real-time data analysis, even during experiments. Gain actionable insights into your material’s behavior through a powerful and versatile set of features seamlessly integrated into TRIOS.
Standard SA Analyses:
- Plot sorption isotherm or isohume (weight change as function of relative humidity or temperature)
- Weight loss on drying
- Weight at specified time, relative humidity or temperature
- Onset and endset analysis
- Step transition analysis
- 1st and 2nd derivative
- Easily import and export SA data with TRIOS
Advanced Analysis Capabilities:
- Exponential and polynomial curve fit
- Sorption kinetics analysis
- Henry, Langmuir, DLP, BET, GAB sorption models
- Advanced custom analysis with user-defined variables and models
 Sorption Isotherm Modeling
Sorption Isotherm ModelingTRIOS is the only software covering the entire workflow from instrument control, data evaluation, modeling of the experimental data with a choice of different isotherm models and report generation. Experimental sorption isotherm data can be easily fitted with a choice of 5 sorption isotherm models. The parameters of the models determined through the data fit can be used to evaluate characteristic material properties, such as the specific surface area. The listing below provides a brief overview of the isotherm models in TRIOS and their characteristics.
Henry Isotherm
One-parameter model providing a linear relationship between RH and sorption. Typically used to describe only the linear part of the isotherm at low RH. The Henry parameter describes the slope of the isotherm in the origin.
Langmuir Isotherm
Two parameter model describing Type I isotherm shape with high uptake at low RH and a saturation at high RH. Parameters describe slope of the isotherm in the origin and the monolayer capacity allowing calculation of the specific surface area of the material.
BET Isotherm
Two parameter model describing Type II isotherms for multilayer sorption not approaching saturation. Parameters describe slope of the isotherm in the origin and the monolayer capacity allowing calculation of the specific surface area of the material.
GAB-Model
Three parameter modification of the BET isotherm extending the range of applicability to higher RH values. Parameters describe slope of the isotherm in the origin, the interaction of adsorbed molecules and the monolayer capacity allowing calculation of the specific surface area of the material.
DLP-Model
Four parameter polynomial model providing superior fitting flexibility for data interpolation. Parameters have no physical meaning.

In a simple-to-apply procedure, TRIOS software determines the parameters of the models to obtain a best fit between the experimental sorption data and the physical or empirical model. The modeling allows users to interpolate between and extrapolate data points as well as to determine isosteric heats of adsorption. Parameters of the physical models provide insight about characteristic properties of the material, such as kinetics of adsorption and BET surface area.
Report Generation
Report Generation
Results can be exported as reports in graphic and numeric form. Predefined format templates can be applied for recurring report generation and help simplify the workflow.
TRIOS Guardian CFR Part 11 Compliance
TRIOS Guardian CFR Part 11 Compliance
Trios Guradian is a fully integrated solution providing CFR 21 Part 11 compliance. It uses the standard file system and does not require maintenance intensive and costly third-party database, hardware, or software. Guardian is designed primarily for laboratories in regulated environments and allows users to establish access limits and system recording protocols. These tools allow compliance with The Electronic Records and Electronic Signatures Rule (21 CFR Part 11) established by the Food and Drug Administration.
Features

- Restricted Access to Authorized Users: The System Administrator can restrict access to various features within the software, which defines the authorized user list. This list is coupled to work with all local and domain-based Windows user accounts.
- User Levels: Authorized users are assigned either a Standard User Level or a Basic User Level. Standard Users have access to the full software functionality.
- Audit Trail: Computer-generated time/date log of events (inherent to the software), including Windows User ID. Also includes the ability to claim authorship.
- Electronic Signatures: Users can electronically sign documents. The signature is added as an entry in the Results Log.
- Results Log: A record of the experimental test conditions and instrument parameters is saved within each data file. Any performed analysis and results functions are also stored within the file.
- PDF File Creation: TA Instruments’ software includes an embedded PDF file generator. This allows any printable document to be saved as a PDF file.
- File Check: Guardian automatically verifies that loaded data files have not been tampered with or modified. If the software detects any modification, the data file does not open and Guardian posts a message in the Notification Log. These events are also recorded in the Audit Trail.
Implementation
- Compatible with all Discovery Series Thermal Analysis instruments.
- Employs the standard TA Instruments software file system. No third-party database, hardware, or software is required.
- Interfaces directly into the PC Windows User Accounts and their associated enforced password policies.
- Specifications
-
Specifications
Dynamic Weighing Range – 1000 mg Weighing Resolution – 0.01 μg Baseline Drift (Standard Deviation) 24 h Isothermal 25° C and 20% RH <±0.25 μg RH-Ramp 5 %– 85% RH at 25° C <±1 μg T-Ramp 25° C to 85° C at 20% RH <±1 μg Sample Temperature – 5° C to 85 °C Humidity Control Range – 0% to 98% RH Humidity Accuracy – ±1% RH Water Refill Pump – Standard Feature Autosampler 10 position Standard Feature 25 position Optional, with platinum or sealed aluminum pans Sample Pans Quartz or Metal-coated Quartz 180 μl Platinum 100 μl Aluminum Sealed 20 μl - Applications
-
Pharmaceuticals
Pharmaceuticals

Water or moisture is commonly found in pharmaceutical products. Water uptake by a drug substance is an inherent property. Raw materials or pharmaceutical products are exposed to water vapor during processing and storage. The efficacy and tolerability of drugs can be altered significantly by the effect of moisture on the active pharmaceutical ingredients and excipients. For this reason, the uptake capacity for moisture must be precisely known. The only way to protect the substances from undesirable changes caused by moisture is to limit the exposure to non-critical levels of humidity.
The United States Pharmacopeial Convention (USP) general chapter <1241> describes water-solid interactions as sorption. The degree of water sorption affects the crystallinity, permeability and melting point of pharmaceuticals. For amorphous materials, the presence of water can significantly alter the bulk properties like glass transition temperature, and may even initiate reversion to the crystalline form. Water also facilitates hydrolysis and induces drug degradation. Although it is not treated as an impurity, water in a drug substance is monitored and controlled as strictly as possible.
Assessing Hygroscopicity
The ability of materials to take up water vapor is often referred to as hygroscopicity. This material property is measured at a constant temperature with changing in RH while weighing the sample mass. These data provide an assessment of the potential effects of moisture on the properties of a pharmaceutical material and serve as criteria in selecting a drug for development. The following table classifies hygroscopicity of pharmaceutical substances as proposed by European Pharmacopeia.
Water sorption data are frequently used in the initial screening process to identify pharmaceutical candidate substances with low moisture uptake.
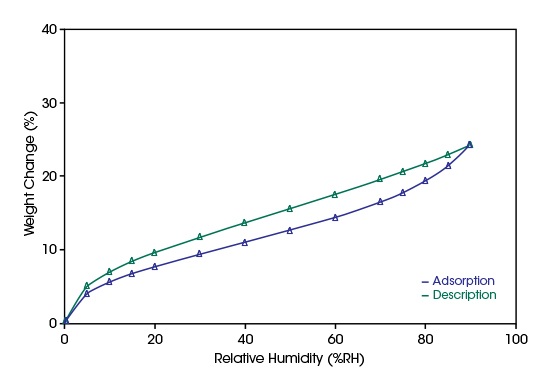
In the diagram below, the adsorption and desorption of water vapor on Ibuprofen at 25°C is shown as function of the relative humidity. According to the classification table, this substance would be considered moderately hygroscopic.
Hygroscopicity Class wt% Water Sorption at 25°C and 80% RH Non Hygroscopic 0 – 0.12 Slightly Hygroscopic 0.2 – 2.0 Moderately Hygroscopic 2.0 – 15.0 Very Hygroscopic >15.0 
Assessing Amorphous – Crystalline Phase Changes
The extent of water vapor sorption depends on the structure of the material. The same material usually absorbs more water if it is in an amorphous state compared to the crystalline structure. Water sorption can lower the glass transition temperature significantly and initiate recrystallization.
An isothermal experiment with ramped RH is useful to identify vapor sorption induced phase transitions. The non-linear moisture absorption of the material indicates the glass transition. Recrystallization leads to a desorption of water with increasing RH. In the diagram below, the weight change of an amorphous lactose sample recorded with ramped RH at 25°C is plotted.

Assessing Hydrate Formation
Approximately one-third of all active pharmaceutical substances (APIs) form hydrates. Spontaneous hydration by moisture in the air can occur at any stage of drug production or storage leading to hydrate formation. The state of hydration changes several properties including physical and chemical stability. Hydrated materials can become amorphous during dehydration, and hydrates affect material solubility, dissolution and bioavailability. Throughout the entire workflow from preformulation to the manufacturing process, packaging and storage, the physical form of excipients and APIs must be fully characterized and controlled. Water vapor sorption is the ideal tool to detect and characterize hydrate formation as a function of temperature and relative humidity.Water vapor adsorption and desorption of anhydrous (AH) sodium naproxen as function of changing RH was investigated in the Discovery SA at 25°C. The stepwise weight changes of the material plotted in the top diagram indicate the formation of monohydrate (MH), dihydrate (DH), and the tetrahydrate (TH).
In the bottom diagram, the results of an isohume measurement at 65%RH with ramping the temperature from 25°C to 50°C are shown. At 25°C the material is in dihydrated condition. With increasing temperature, it dehydrates into a monohydrate which is completed at temperatures higher than 45°C.


Polymers
Polymers
Polymeric materials are applied widely in manufacturing of consumer products and as packaging material. Many polymers have a natural tendency to absorb water from their humid environment. Absorbed water has been shown to act as a plasticizer, reducing the glass transition temperature and mechanical strength. However, absorbed water also can lead to irreversible degradation of the polymer structure.
Gravimetric vapor sorption measurements are suggested in ASTM, ISO and other technical standards for evaluating polymer water interactions. The Discovery SA measures the water absorption as weight increase of a polymer material exposed to a controlled RH allowing to assess the hygroscopic stability of the material. The kinetics of water uptake or release is characteristic for the water permeability of the polymer material and can be extracted from the continuously recorded weight data.
Hydrolysis Stability of Polymers for Electronic Devices
In electronic device manufacturing, reliability issues associated with water absorption have become increasingly important. Advanced polymer based materials are applied for more function integration and further miniaturization. Their properties must remain intact when they are exposed to environmental humidity.
Kapton is a polyimide polymer that remains stable in dry conditions in a wide temperature range. Kapton is used as base material for printed circuits for flexible electronics and as an insulation and protection layer on electrostatic sensitive and fragile components. The improved electrical, chemical, and mechanical properties of Kapton compared to other commonly used polyimide materials are a result of its significantly improved hydrolysis resistance.
In the diagram below, water vapor ab- and desorption data measured on a Kapton tape at 25°C are plotted. As expected, the water vapor sorption is found to be small compared to other polyimide polymers.

Assessing Water Sorption of Fuel-Cell Membranes
Improvements in electrochemical conversion of water have been achieved through the development of new proton exchange membrane materials (PEM). Conversion of hydrogen and oxygen into water in fuel cells relies on a PEM. The same is true for the conversion of water to hydrogen and oxygen in electrolysers. In both, the PEMs form the heart of the electrochemical cells and understanding the mechanisms of their degradation allows researchers to develop more reliable and effective fuel cells and electrolysers.In the plot below, the water vapor ab- and desorption on a perfluorosulfonic acid membrane is compared for 25°C and 80°C. While the amount of water sorption is almost unchanged, the hysteresis between ad- and desorption disappears at the higher temperature. This indicates a higher reversibility of water sorption at higher temperatures improving the removal of the reaction product from the membrane material.

Water Permeation in Packaging Polymer Films
The first step of water permeation through a polymeric packaging material is the absorption of moisture from the environment. A low water vapor absorption capacity and/or a slow kinetics of ab- and desorption indicate a low permeability. Water vapor sorption is an extremely valuable tool for comparing polymeric films used for packaging drugs and other humidity sensitive products.The plot below compares sorption kinetics for two different polymeric packaging films undergoing temperature and relative humidity cycling. Film A absorbs and desorbs moisture at a more rapid rate than the other film. Higher sorption capacity and faster sorption kinetics suggest that film A is less suitable for packaging moisture sensitive materials than film B.

Assessing Hydroscopicity of Natural Polymers
Microcrystalline cellulose (MCC) is a naturally occurring polymer. MCC is a valuable additive in pharmaceutical, food, cosmetic and other industries. Amongst other properties of MCC, moisture sorption capacity and moisture content are measured to qualify its suitability to such utilization.
In the diagram, the moisture sorption isotherm data of MCC are shown together with a fit of the data with the GAB and the DLP models. While the parameters of the DLP model have no physical meaning, the GAB parameter characterizing the monolayer sorption capacity Wm = 2.2×10-3 mol/g allows the calculation of the specific surface area of the material:
SA = Wm×N×AW with N = 6.0221×1023 molecules/mol and AW = 12.5×10-20 m2/molecule
SA,MCC = 166 m2/g

Food
Food

Moisture content is a critical factor to consider in the food industry. The amount of water in a product affects the product’s texture, shelf life, ease of processing, and cost to produce. Increased moisture content of food can make crispy food products soft or fresh pasta sticky and difficult to manage. On the other hand, if the product is too dry, lack of moisture can make it brittle, or hard as a rock. Further, microbial activity increases with the moisture availability in the food. Moisture rich foods are easily susceptible to microbial attacks, rot, and damage. Thus, the shelf life of the food material is determined by the moisture content in the food.
Developing suitable recipes and optimal processing and storage conditions allows manufacturers to control the moisture absorption of food from the atmosphere. Well conditioned foods with controlled moisture sorption preserve the taste and desired texture, provide a better shelf life and enhance the customer experience.
Assessing shelf Life and Storage Stability
Crispness is one of the most important sensory quality attributes of corn flakes. It should be preserved when the corn flakes are stored after the pack is opened. This requires low moisture sorption at lower humidity levels. At high humidity, the moisture sorption should increase considerably to allow the milk to penetrate the flake before consumption.
In the diagram below, water vapor ab- and desorption data measured on corn flakes at 25°C and 40°C are compared. The sorption isotherms exhibit at both temperatures the desired type III shape with low moisture sorption up to 40%RH. This indicates that in the investigated range, temperature has no significant influence on the storage stability of corn flakes.

Assessing Hydroscopicity of Corn Starch
Starch is one of the most important bio-polymer components of cereals, which determines to a great extent their hygroscopic properties. Starch is also used in many food products, whose preservation depends essentially on its humidity sorption properties. Owing to its versatile properties and variability, starch is also used in the production of packaging materials, biotechnology, fragrance, textile, and pharmaceutical products.In the diagram below, the ab- and desorption of water vapor in corn starch measured at 25°C is plotted as function of RH. The continuous progressing sorption isotherm type II and the relatively small hysteresis are characteristic for corn starch.

Construction Materials
Construction Materials

The moisture absorption properties of building materials are critical to improving durability, designing low-energy building structures, and effective impregnation. Ultimately, materials with controlled moisture sorption properties are critical for resident comfort and well-being.
Humidity or moisture is considered one of the highly relevant factors to the reliability and proper functioning of building structures. In particular, for building materials moisture sorption has significant implications for stones, cements, woods, and insulation materials. Moisture damage is a significant factor limiting a building’s lifespan. As well, moisture infusion through a building’s outer structure can have a significant effect on indoor air quality and air-conditioning load.
The water vapor sorption isotherm is one of the main parameters necessary for the analysis of material’s hygroscopicity and moisture transport between the building environment and the indoor air.
Assessing Water Sorption in Wood
Wood is a key natural resource and a versatile material used in building and construction applications. Its structural properties vary with the water content and it is subject to natural decay processes. Therefore, it is necessary to understand the water sorption in wood as a function of humidity.Protection of wood can be achieved by preventing water transport across the material, through sealing the surfaces and treating the wood so that it does not absorb moisture. The analysis of the susceptibility of wood to natural decay and the suitability of wood for construction can be performed through vapor sorption measurements.
In the diagram, the ad- and desorption of water vapor on three plywood samples of different densities are compared.

Adsorbents and Catalysts
Adsorbents and Catalysts
 Developing water tolerant adsorbent materials and adsorption processes is essential for cost and energy efficient purification and gas storage processes. Measuring water vapor adsorption isotherms on the materials is key information for improving their properties. Adsorbent materials are used in a wide range of industrial and environmental applications, including purification and separation of mixtures, drying, catalysis, pollution control and others. Most materials have a porous texture with a high specific surface area. In many separation applications – except in drying – water is not considered a pollutant which should be adsorbed. Adsorbed water blocks adsorption capacity and reduces the efficiency of the material. Some adsorbents, such as novel metal-organic networks (MOF), which excel in gas storage and purification due to their very high porosity, are not stable in the presence of water.
Developing water tolerant adsorbent materials and adsorption processes is essential for cost and energy efficient purification and gas storage processes. Measuring water vapor adsorption isotherms on the materials is key information for improving their properties. Adsorbent materials are used in a wide range of industrial and environmental applications, including purification and separation of mixtures, drying, catalysis, pollution control and others. Most materials have a porous texture with a high specific surface area. In many separation applications – except in drying – water is not considered a pollutant which should be adsorbed. Adsorbed water blocks adsorption capacity and reduces the efficiency of the material. Some adsorbents, such as novel metal-organic networks (MOF), which excel in gas storage and purification due to their very high porosity, are not stable in the presence of water.Water Adsorption on Hydrophilic Absorbents
Zeolites are microporous, aluminosilicate minerals bearing a negatively charged honeycomb framework of micropores into which molecules may be adsorbed. Zeolites occur naturally but are also produced industrially on a large scale. A-type zeolites are used industrially in natural gas drying and desulfurization as well as separation of nitrogen and oxygen.
Due to their polar nature, instantaneous water adsorption happens at a low RH level. This behavior can be observed as the typical steep type I isotherm in the diagram. The Discovery SA can control RH in small increments, so that the steep increasing branch of the isotherm can be analyzed.

Water Adsorption on Hydrophobic Absorbents
Activated carbons are the most widely used industrial adsorbent for removing contaminants and pollutants from gaseous, aqueous, and nonaqueous streams. They are inexpensive to manufacture and have uniquely powerful adsorption properties. Depending on the starting material and the activation process, a wide range of pore structures can be generated which makes porous carbons applicable to a wide range of technologies. Their non-polar interface leads to weak interactions with water vapor. As a result, the isotherm is of type III with a low uptake at low RH levels. The increasing water sorption at higher RH levels is due to pore condensation. Between the adsorption and desorption branch of the isotherm, a hysteresis is formed which is characteristic for the pore size distribution of the activated carbon.

CO2 Capture
CO2 Capture
Gas blending and humidity control providing flexibility to assess carbon capture materials
Humidity sorption at controlled CO2 levels
The blending GDM can be programmed to control a constant CO2 concentration while a sorption experiment with ramped of stepped humidity is performed. In this example humidity sorption on a microporous activated carbon is measured at different CO2 concentrations. In the diagram to the right the humidity uptake from a pure N2 carrier gas is compared to the uptake measured when 10% or 20% CO2 were blended in the carrier gas. As the general type III shape of the humidity isotherms remains unchanged, the water sorption is reduced with increasing CO2 concentration in the carrier gas. This indicates the strong affinity of the material for CO2 which leads to the lower water sorption.
CO2 sorption at controlled RH
The blending GDM can also be programmed to control a stepwise change or ramping of the CO2 concentration. In this modus CO2 adsorption and desorption isotherms can be measured at controlled humidity. In this example, CO2 sorption on a microporous activated carbon is measured at different RH levels. In the diagram to the right, the CO2 uptake from dry carrier gas is compared to the uptake measured at 20% or 40% RH levels. The type I shape of the CO2 isotherms is obvious at all RH levels. The CO2 uptake is slightly reduced with increasing RH. This indicates the co-adsorption of humidity despite the strong affinity of the material for CO2 leading to the reduced CO2 sorption.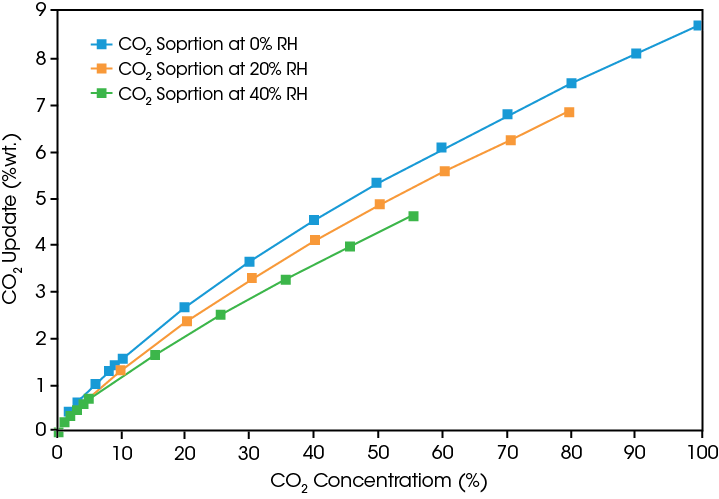
- Video
-
















