Keywords: DMA, gelatin, capsules, pharmaceuticals
TA409
Abstract
Pharmaceutical drug capsules are mostly made of gelatin. Gelatin is a very hygroscopic material. The thermal and mechanical properties of these kinds of gelatin based capsules are very sensitive to both temperature and humidity. In this paper, the humidity influence to the thermal mechanical properties of gelatin capsule was studied systematically using TA Instruments’ Dynamic Mechanical Analyzer with the humidity control unit. The coefficient of hygroscopic expansion (CHE) of the gelatin capsule was also studied at two individual temperatures.
Introduction
Gelatin is a partial hydrolysis product from a highly structured protein – Collagen. Depending on the source of the collagen protein (i.e. bovine, porcine, poultry or fish), the processed gelatin may exhibit dramatic different physical properties [1-3]. Gelatin can be water soluble at certain high temperatures. When being cooled down, gelatin forms a hydrogel with different strength depending on its water content. In general, gelatin is graded primarily on the strength of the gel it forms [4]. Due to its nontoxic nature and unique visco-elastic physical properties, gelatin has been widely used in pharmaceutical area, specifically for making drug capsules. The gelatin based capsules are typically composed of gelatin, a plasticizer or a combination of multiple plasticizers and water. In addition, they may also contain preservatives, coloring and opacifying agents or sweeteners. The water content in a gelatin capsule varies from 10-16%. The amount of water in the capsule plays an important role on its physical properties [5-6]. The mechanical strength of the capsule, the glass transition (Tg) temperature and even the shelf life of the capsule are all affect by the water content. Gelatin is hygroscopic. It can absorb moistures from the air. High humidity may cause the gelatin to undergo extensive cross-linking during storage, which will affect it dissolution and swollen. This will further affect the drug release inside the capsule [7-9]. Therefore, it is important to better understand the humidity influence to the thermal and mechanical properties of the gelatin based drug capsules.
The thermal properties of gelatin capsule have been studies using varieties of different techniques. DSC and modulus DSC have been used to determine performance related microstructural features such as the glass transition temperature (Tg), the melting temperature (Tm) and the melting enthalpy (Hm) [10-12]. Tg, Tm and Hm are important parameters for monitoring process and storage induced structural changes and have been successfully applied to the design and optimization of the gelatin capsule formulation. However, there are limited researches on studying the humidity influence to the thermal and mechanical properties of the capsules. TA Instruments DMA with the humidity test chamber (shown in figure 1) is a powerful instrument which allow of analyzing the thermal and mechanical properties of gelatin capsule and other materials under both temperature and humidity control. It is equipped with varieties of clamp fixtures, which allows samples to be measured with different dimensions. The humidity control chamber is good for the temperature over the range of 5-120 °C and the humidity over the range 5% to 95% RH. This is ideal for analyzing the property changes of gelatin capsule over its applications conditions. The objective of this study is to investigate the thermal and mechanical properties of gelatin capsule including glass transition (Tg), moduli (E’ and E”), and its coefficient of hygroscopic expansion (CHE).

Experimental
The gelatin capsule (size 000) is obtained from Torpac Inc. Based on the manufacturing report, this capsule is composed of gelatin (85% – 86%); de-ionized water (13% – 14%); methyl paraben (0.75%); propyl paraben (0.14%); and sodium lauryl sulphate (0.14%). The capsule is cut into strips with a dimension of about 8-10 mm long and 1-2 mm wide. The thickness of the capsule is reported to be 0.1 mm.
A TA instruments dynamic mechanical analyzer with the humidity control chamber was used for all of the property tests. The sample specimen was loaded to the film tension clamp fixture (shown in figure 2). Based on the common application and storage conditions, the gelatin capsule samples were studied with the following methods.

1. Hold temperature constant, ramp the humidity
The gelatin specimen was loaded at ambient condition. Then it was equilibrated in the chamber to the initial measurement temperature (i.e. 25 °C; 37 °C; 50 °C respectively). After equilibration, held the temperature constant and ramped the relatively humidity from 0% to 90% at a rate of 2%/min. Held humidity at 90% for additional 15 minutes. A dynamic oscillatory test was conducted during the test at a frequency of 1 Hz and strain amplitude within the linear viscoelastic region of the material. The sample moduli (E’ and E”) and tan delta parameters were recorded.
2. Hold humidity constant, ramp temperature
The gelatin specimen was loaded at ambient condition. Then it was equilibrated in the chamber to the initial measurement temperature (20 °C) and the initial relative humidity (i.e. 5%; 50%; 60% and 80% respectively). After the equilibration, held the humidity constant and ramped the temperature from 20 °C to 100 °C at a rate of 0.5 °C/min. A dynamic oscillatory test was conducted at 1 Hz of frequency to monitor sample modulus change.
3. Step the humidity to measure the Coefficient of Hygroscopic Expansion (CHE)
The gelatin specimen was loaded at ambient condition. A static load of 0.01 N was applied to the specimen. Then it was equilibrated in the chamber to the initial measurement temperature (i.e. 25 °C; 37 °C and 50 °C respectively). The initial sample length was recorded at the end of the equilibration. After the equilibration, the relative humidity was increased with 10% RH per step from 10% RH up to 90% RH. The sample was equilibrated at each humidity step for 2 hours until the sample displacement did not show any further change. The sample displacement at each humidity step was recorded for the calculation of sample CHE.
Results and Discussions
1. Humidity influence to the glass transition of the gelatin capsule
Glass transition is associated with the polymer chain movements. It is an important parameter for evaluating material’s storage and operation conditions. Below the glass transition, the material shows high stiffness and modulus. After the transition, the storage modulus drops about one to several decades and the material goes into a rubbery state. In general, the glass transition of a material is very sensitive to temperature. If the material is hygroscopic, its glass transition will also be sensitive to the relative humidity of the environment.
Figure 3 shows the humidity ramp test results of gelatin capsule at 25 °C; 37 °C and 50 °C. The storage modulus of the sample was about the same at 4500 MPa under low humidity below its glass transition. Based on the loss modulus curve, two transitions were observed during the humidity ramp. The first transition showed at humidity of 51% – 55% was weak and broad. This transition was not very temperature sensitive. At 25°C, this transition peak showed at 51% RH. And at 50 °C, it showed at 55% RH. The second transition showed at humidity from 78% up to 90%. This is the glass transition of the gelatin capsule. It is obvious from the test results that this transition is sensitive with the measurement temperature. The higher the temperature, the transitions showed at lower humidity level. In addition, the storage modulus of the sample underwent significant decrease during this transition. These results provide important information about the glass transitions of gelatin based capsule at storage temperatures.
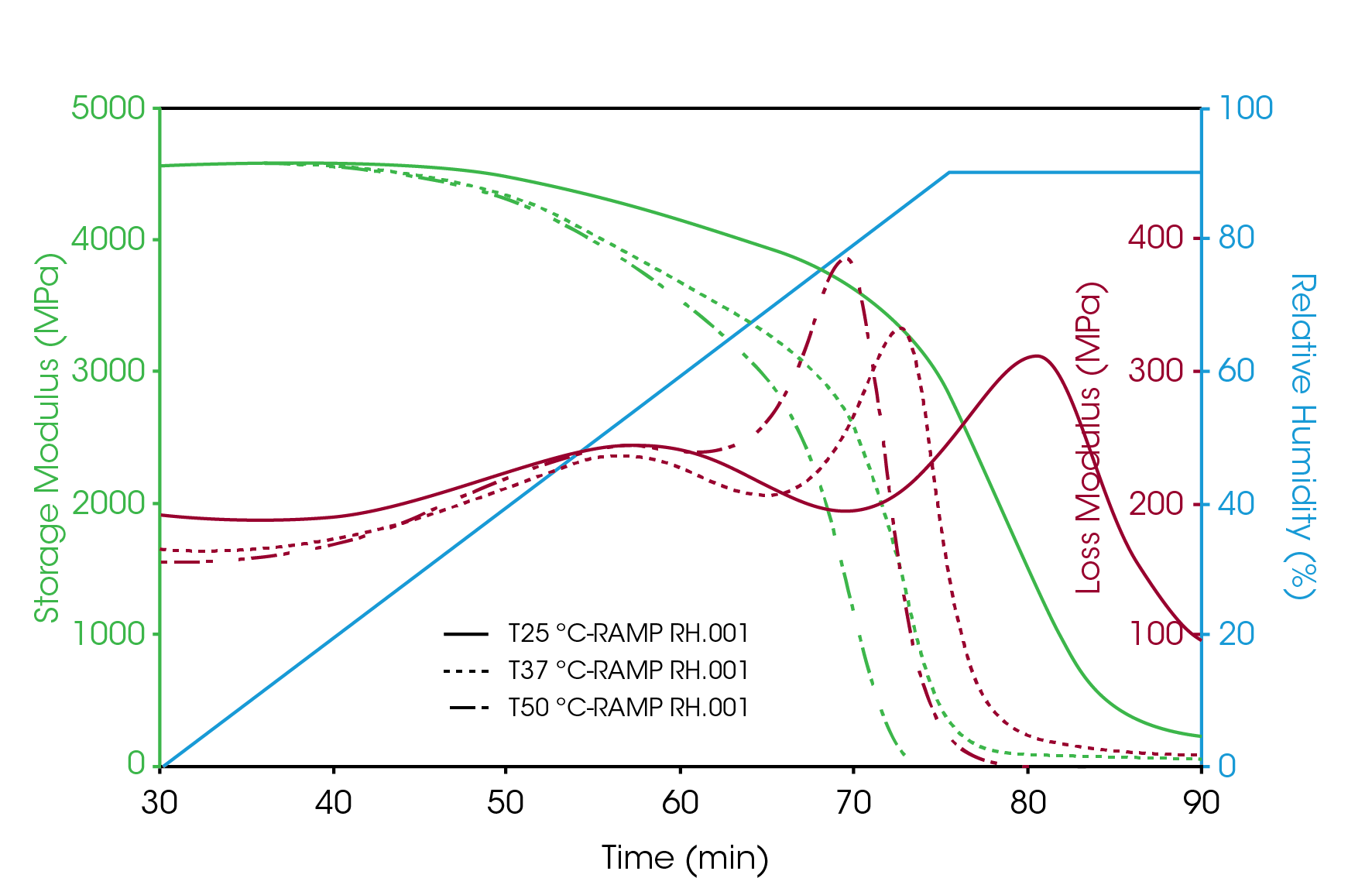
2. Humidity influence to the mechanical strength of the gelatin capsule
As reported by the manufacturer, the water content of the gelatin capsule is about 13%. However, since gelatin is hygroscopic, it has the capability to absorb additional water from the environment. The additional amount of water can significantly lower the glass transition temperature and the modulus of the capsule. Figure 4 shows the temperature ramp test results of gelatin capsule at different relative humidity. When being stored at relatively dried condition (e.g. RH=5%), gelatin capsule can remain stable over a broad temperature range. As the humidity of the environment increased up to 50%, the storage modulus of the capsule starts to decrease at temperature higher than 40 °C. At 60% humidity, the storage modulus of the capsule at 20 °C is only 50% compared with its dried condition. And it keeps dropping as temperature increases. At temperature higher than 35-40 °C, the modulus of the capsule has dropped more than one decade and cannot be used as usual. These experimental results indicate that storage humidity condition is important for the mechanical properties and shelf life of the gelatin capsules.
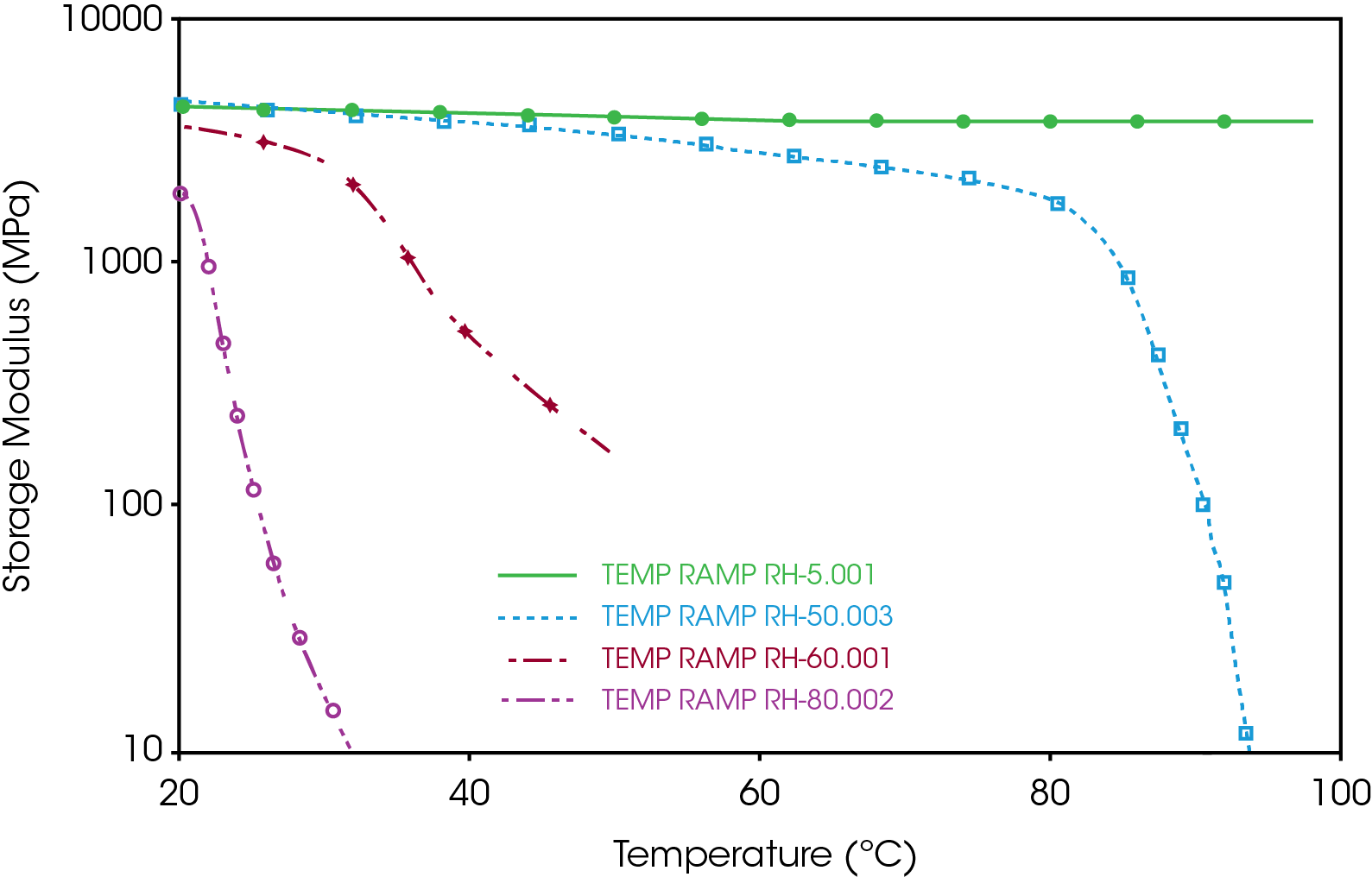
3. The CHE of the gelatin capsule
The coefficient of thermal expansion (CTE) of a material is commonly measured using a TMA instrument. This CTE evaluates the dimension change of a material as a function of temperature change. However, for a hygroscopic material, its dimension is also sensitive to the environment humidity change. Therefore, understanding the coefficient of hygroscopic expansion (CHE) is also important for evaluating the physical properties of a hygroscopic material.
Figure 5 shows the dimension measurement results of gelatin capsule at 37 °C and different humidity level. Humidity equilibration is time consuming. The results from figure 5 show that for a gelatin capsule with thickness of 0.1 mm, it takes at least 2 hours for the sample to reach equilibration at each humidity step. The sample length was measured accurately at the end of each humidity step. The coefficient of hygroscopic expansion (CHE) is obtained by taking the slop of sample displacement vs. relative humidity. Figure 6 shows the CHE of gelatin capsule measured at 2 different temperatures.
As can be seen from the humidity step test results in figure 5, at 37 °C and 80% relative humidity, gelatin capsules undergo a significant shrinkage in dimensions. This indicates that at 37 °C, the gelatin capsule is unstable being stored at humidity greater than 80%.
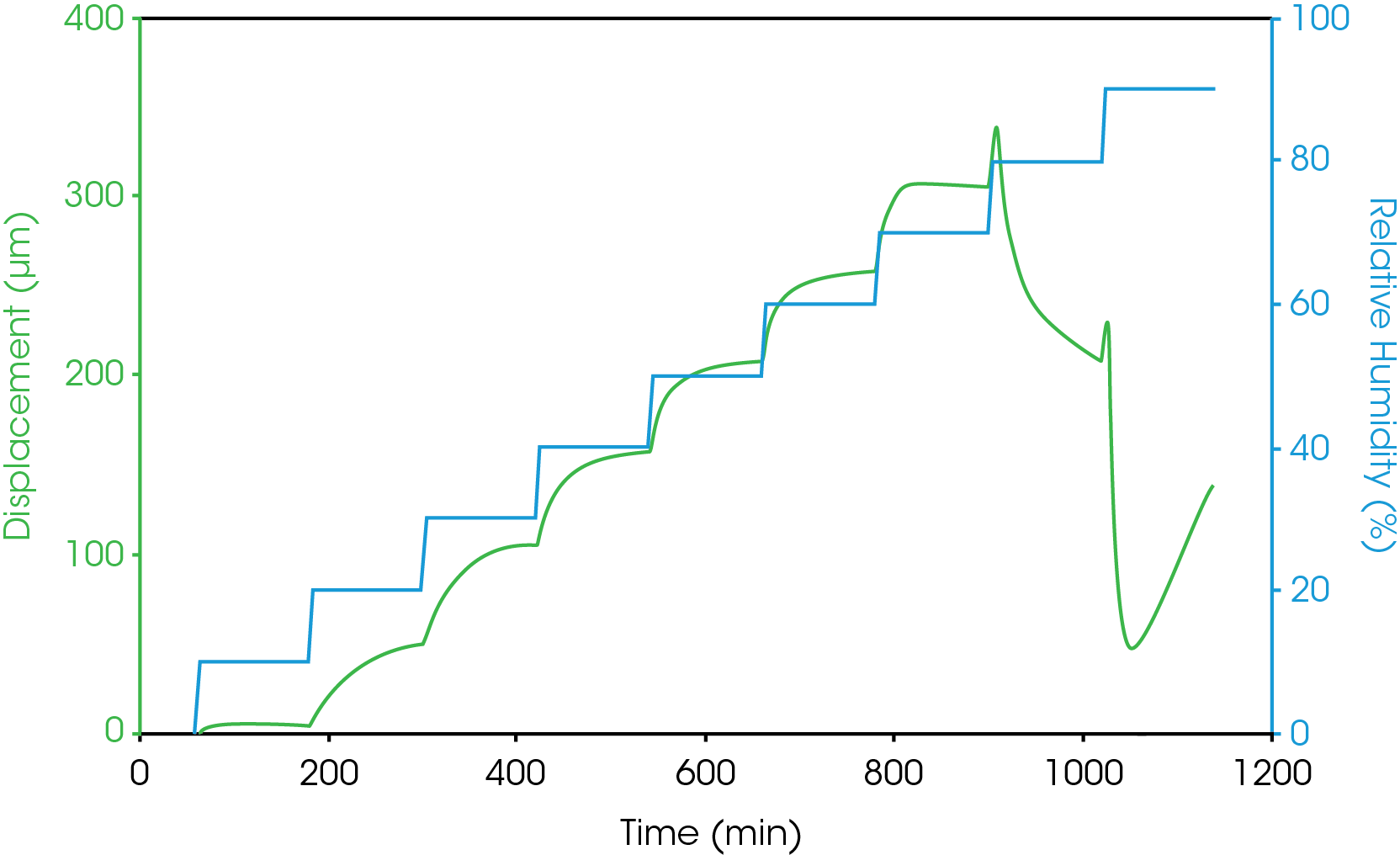
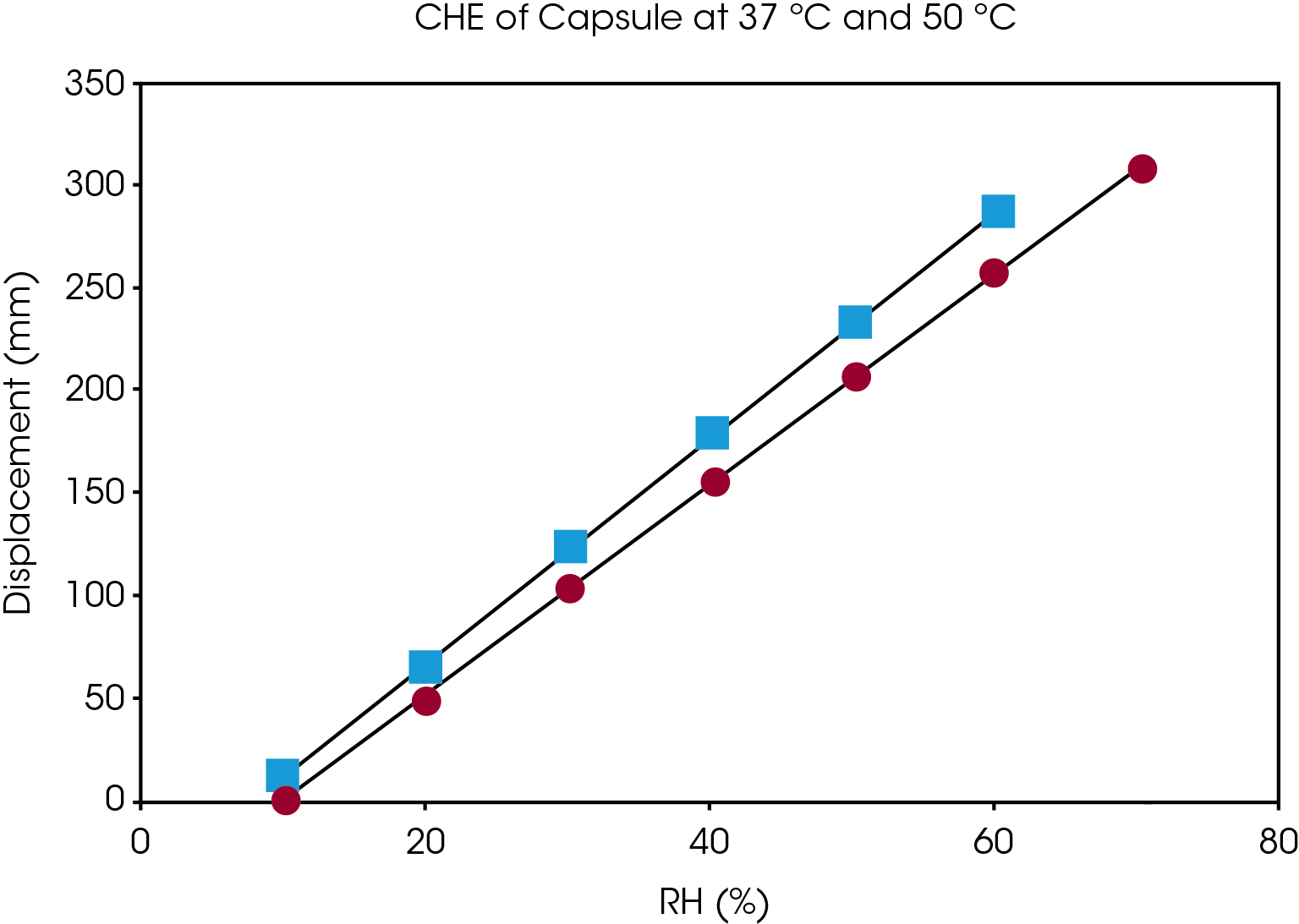
Conclusions
Due to its nontoxic nature, gelatin is commonly used in pharmaceutical industries. One major application is for the making of drug capsules. Over the years, many researches have been done to investigate the chemical and physical properties of the gelatin materials. However, since gelatin is hygroscopic, the water content in gelatin, the moisture level in the environment also plays an important role for the physical behavior of the gelatin capsules. TA Instruments DMA with the humidity control chamber provides a powerful technique for analyzing the thermal and mechanical properties of gelatin capsules under both temperature and humidity control. The experimental results show that the glass transition and the mechanical strength of gelatin capsule varies significantly with the environment temperature and humidity. In addition, the DMA can also measure the coefficient of hygroscopic expansion of gelatin capsule. These studies provide important information for the gelatin capsule manufacturing and storage.
References
- Ward, A.G.; Courts, A. “The Science and Technology of Gelatin”. New York: Academic Press. ISBN 0127350500.(1977)
- Herbert Gareis; Reinhard Schrieber. “Gelatine Handbook: Theory and Industrial Practice”. Weinheim: Wiley-VCH. ISBN 3- 527-31548-9. (2007)
- Mayo, K. H. Biopolymers (Peptide Sci) 1996, 40, 359–370.
- British Standards Institution. “Methods for Sampling and Testing Gelatine”. 2 Park St. London W1A 2BS. BS 757: (1975)
- Kontny MJ; Mulski CA. “Gelatin Capsule Brittleness as a Function of Relative Humidity at Room Temperature”. Int. J Pharm., 1989; 54, 79-85.
- Marques MRC; Cole E; Kruep D et al. “Liquid-filled Gelatin Capsules”, Pharmacopeial Forum. 2009; Vol 35(4), 1029-1041
- Murthy KS, Ender NA, Fawzi MB. “Dissolution Stability of Hard-shell Products. Part I. The Effect of Exaggerated Storage Conditions”. Pharm Technol. 1989; 13: 72-84
- Murthy KS, Reisch RG, Fawzi MB. “Dissolution Stability of Hard-shell Products. Part II. The Effect of Dissolution Test Conditions on in Vitro Drug Release”. Pharm Technol. 1989; 13: 53-58
- Ofner CM III, Zhang Y, Jobeck VC, bowman BJ. “Crosslinking Studies in Gelatin Capsules Treated with Formaldehyde and in Capsules Exposed to Elevated temperature and Humidity”. J Pharm Sci. 2001, 90 (1) 79-88.
- Nazzal S, Wang Y. “Characterization of soft gelatin capsules by thermal analysis”. Int. J. Pharm. 2001, 230, 35-45
- Tseretely GI, Smirnova OI. “DSC Study of Melting and Glass Transition in gelatins”. J. of Thermal Analysis. 1992, Vol 38, 1189-1201
- Dai CA, Chen YF, Liu MW. “Thermal Properties Measurements of Denatured Gelatin using Conventional and Temperature Modulated DSC”. J of Applied Polymer Science. 2006, 99, 1795-1801
Acknowledgement
This paper was written by Tianhong (Terri) Chen, Ph.D. Applications Specialist at TA Instruments
Click here to download the printable version of this application note.

