Keywords: Powder rheology, Pharmaceutical, Excipient, Drug formulation, Tableting, Lactose, CMC
RH140
Abstract
Powder rheology is essential in the pharmaceutical industry for the characterization of excipients and the optimization of drug formulations. It involves the study of the flow behavior and deformation of powders, which directly impacts manufacturing processes and the quality of the final product. The TA Instruments™ HR Powder Rheology Accessory measures flowability, compressibility, and shear with temperature control, providing a complete picture of powder flow behavior and particle interactions. Lactose monohydrate and carboxymethyl cellulose (CMC), popular excipients in the pharmaceutical industry, have rheological properties such as flowability, cohesion, and compressibility. These properties are quantified to optimize formulations before scale-up.
In this study, lactose exhibited over three times higher compression and cohesion values compared to CMC. Both powders showed rate-dependency, with flow energy decreasing as tip speed increases. Their rheological properties were also examined at 45 °C, simulating exposure to sunlight, and at 4 °C, simulating refrigeration. At 4 °C, the flow function of lactose monohydrate shifted towards cohesive flow.
It can be concluded that under high stress, CMC flows better, while under moderate to relatively low stress, lactose monohydrate flows better. Under very low to no stress, both have similar flowability. Lactose monohydrate demonstrates easy flow, relatively low confined flow energy, moderate cohesion, and excellent compressibility. CMC exhibits lower cohesion but higher confined flow energy, which should be taken into account when considering CMC as a filler.
Introduction
The rheological properties of powders are vital for ensuring uniform die filling during tablet production. Poor flow can result in inconsistent tablet weights and content uniformity issues. High cohesion can lead to problems such as sticking and picking during the tableting process [1-8]. Rheological measurements help optimize formulations before scale-up by considering factors such as flow behavior, compressibility, and the shear properties of consolidated powder under varying temperature conditions. Additionally, understanding powder rheology is crucial for designing hoppers and feeders that ensure uniform flow into the dies [7,8].
The choice of excipients, either as binders or fillers, is critical as they impact both formulation and tablet production. Excipients enhance the flow properties of the powder blend, ensuring consistent die filling and tablet weight. They aid in forming tablets that are robust enough to withstand handling yet disintegrate appropriately in the body. Binders must provide sufficient cohesion and compressibility without making the powder overly sticky. As binders, excipients ensure that the powder particles adhere to each other, forming a cohesive and strong tablet. Excipients as fillers, on the other hand, should improve flowability, ensuring that the powder can be easily processed and uniformly filled into mold and dies. Additionally, fillers help achieve the desired tablet size and shape, making it easier to handle [1-4,6,7,9]. These small-scale laboratory measurements are essential for avoiding expensive optimization processes at full industrial scales and for fully leveraging quality-by-design approaches in pharmaceutical development.
Experimental
Materials
Lactose monohydrate (Loudwolf Industrial and Scientific) and carboxymethyl cellulose (NEI corporation) were examined in this study. All measurements were performed using the Discovery™ HR-30 Rheometer and the Powder Rheology Accessory with interchangeable shear and flow cells (shown in Figure 1 and Figure 2). Experiments were run at varying temperature conditions and ambient relative humidity of 46%.

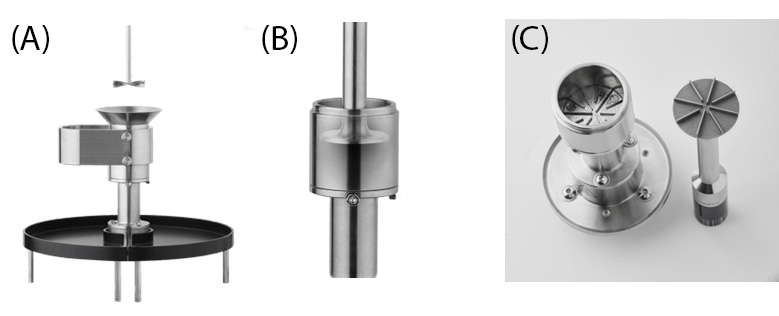
Powder Flow Cell with temperature control
The Powder Flow Cell was utilized to measure flowability by moving an impeller rotor through a powder bed in a helical path (see Figure 2A). Unconfined flow was measured as the impeller moved upward through the powder, while confined flow was measured as the impeller moved downward [1,3]. Before testing, the powders were loaded into the cup and conditioned by moving the impeller up and down through the powder in two stages, first at a tip speed of 100 mm/s and then at 60 mm/s. After conditioning, the powders were trimmed, as shown and detailed in RH126 [2]. This procedure ensured uniformity between test samples and improved the reproducibility of results.
After conditioning, flowability tests were conducted at tip speeds of 150, 100, 60, 30, and 10 mm/s to assess confined and unconfined flow and their rate dependency. This process was repeated for seven measurement cycles using fresh samples for each tip speed to ensure data reproducibility. Then, normal force and torque were measured to determine flow energy.
Powder Compressibility with temperature control
A Powder Compressibility test is used to characterize the compressibility of powders by implementing a flat upper plate as the compressibility geometry and the same flow cup from the Powder Rheology Accessory (see Figure 2B). The Powder Compressibility step applies a sequence of increasingly larger normal stresses to the powder with zero applied torque. It equilibrates at each normal stress increment for a user-defined increment to wait for the gap to reach a steady state (measuring the change in gap) to derive a relationship between the applied normal stress and powder volume (bulk density). Following the same flow conditioning step as previously described, the initial gap value is set to 30 mm. For compressibility steps, normal stress is then incrementally applied in steps of 2 kPa, up to a maximum of 20 kPa. Each increment is maintained for 60 seconds to allow the gap value to stabilize.
Powder Shear Cell with temperature control
Shear measurements under consolidation stress were conducted using the Shear Cell accessory, shown in Figure 2C. This accessory features a serrated upper plate and cup to prevent slippage during the shearing of compacted powder. The powders were loaded using the provided loading slide and funnel, then consolidated by applying a chosen stress level of 6 kPa and 15 kPa for this study. Excess material was trimmed to level the surface for testing.
Testing followed ASTM D7891 (Standard Test Method for Shear Testing of Powders). Each consolidation normal stress involved a pre-shear step, maintaining the consolidation stress on the powder sample while slowly rotating it at an angular velocity of 1×10-3 rad/sec for standard mode and 5×10-4 rad/sec for precision mode until the measured shear stress reached a steady state. Subsequently, the normal stress was reduced, and rotation continued until the powder exhibited incipient yield, indicated by a peak in shear stress. The pre-shear step was repeated with the same initial normal stress to ensure consistent powder consolidation conditions, followed by a shear measurement under a lower normal stress [1,3]. For various tests, a different number of cycles, ranging from 5 to 10, were performed under normal stresses between 12 kPa and 3 kPa.
The rheological properties of lactose monohydrate and CMC, including compressibility, flowability, and shear measurements, were examined at room temperature, 45 °C to mimic the effect of sunlight exposure, and at 4 °C to simulate the influence of refrigeration.
Results and Discussion
Compressibility
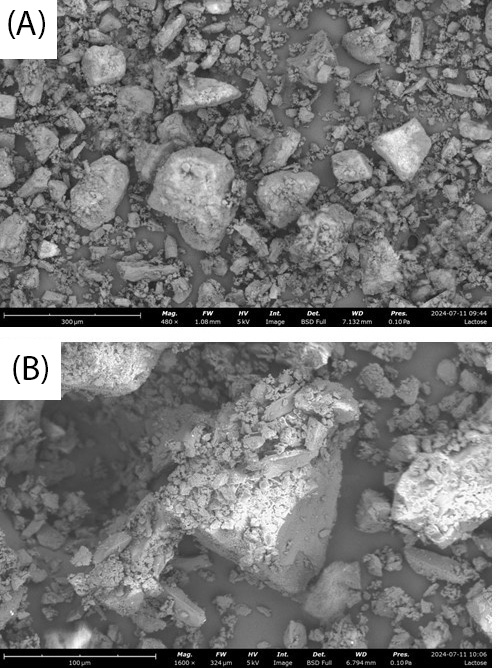
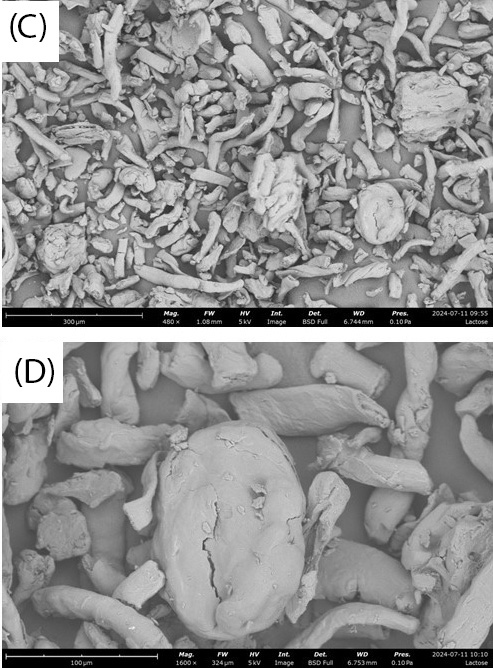
Lactose monohydrate exhibits over three times higher compressibility than CMC, as shown in Figure 3 and Figure 4. When there is a wide distribution of particle sizes, larger particles can create voids that smaller particles effectively fill, leading to higher compressibility. SEM images in Figure 5A and Figure 5B suggest this phenomenon for lactose monohydrate. Conversely, needle-shaped or platelet-shaped particles tend to have lower compressibility because their form creates more void spaces. This could explain the lower compressibility of CMC, as observed in SEM images in Figure 5C and Figure 5D [10].
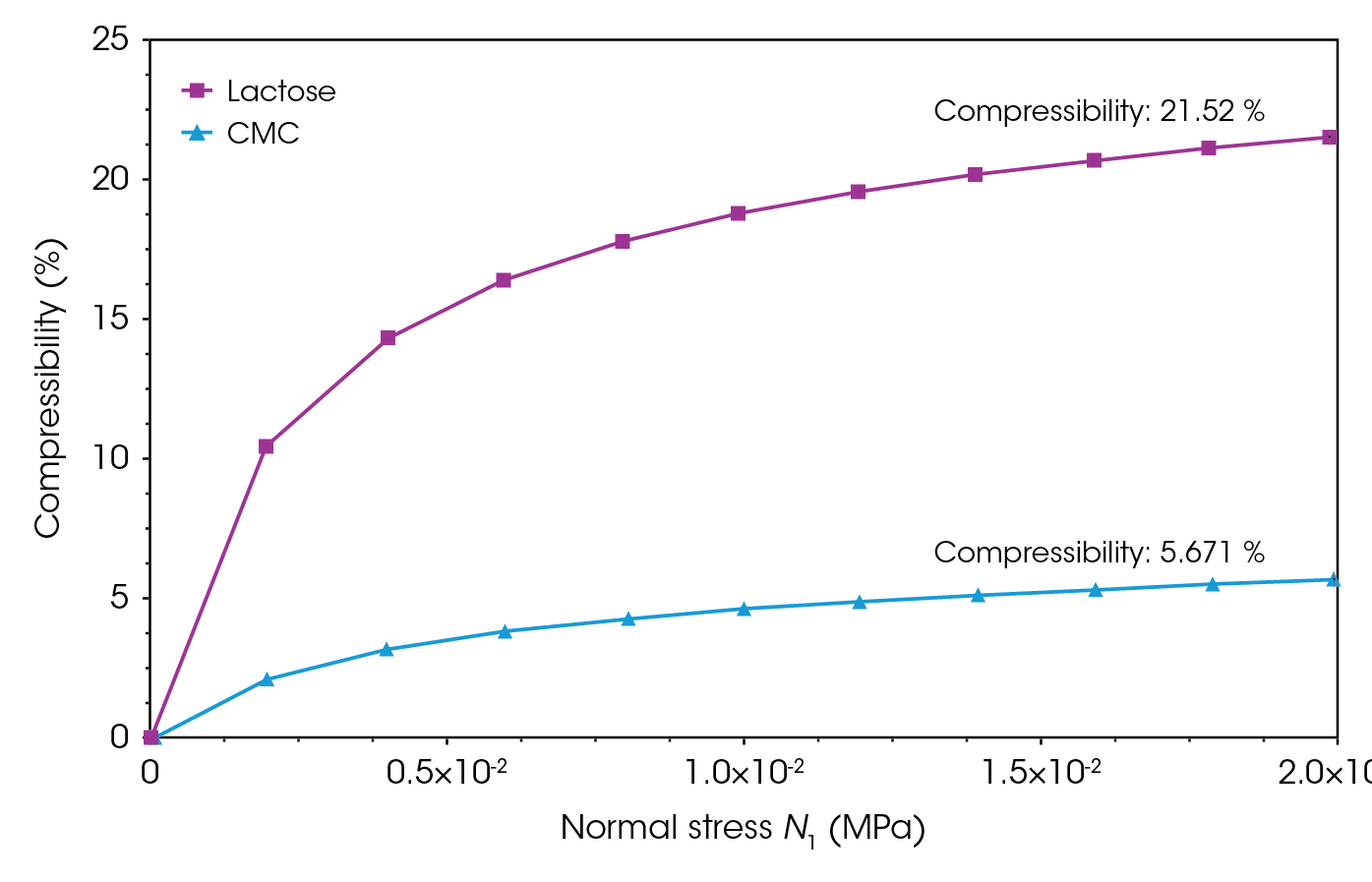
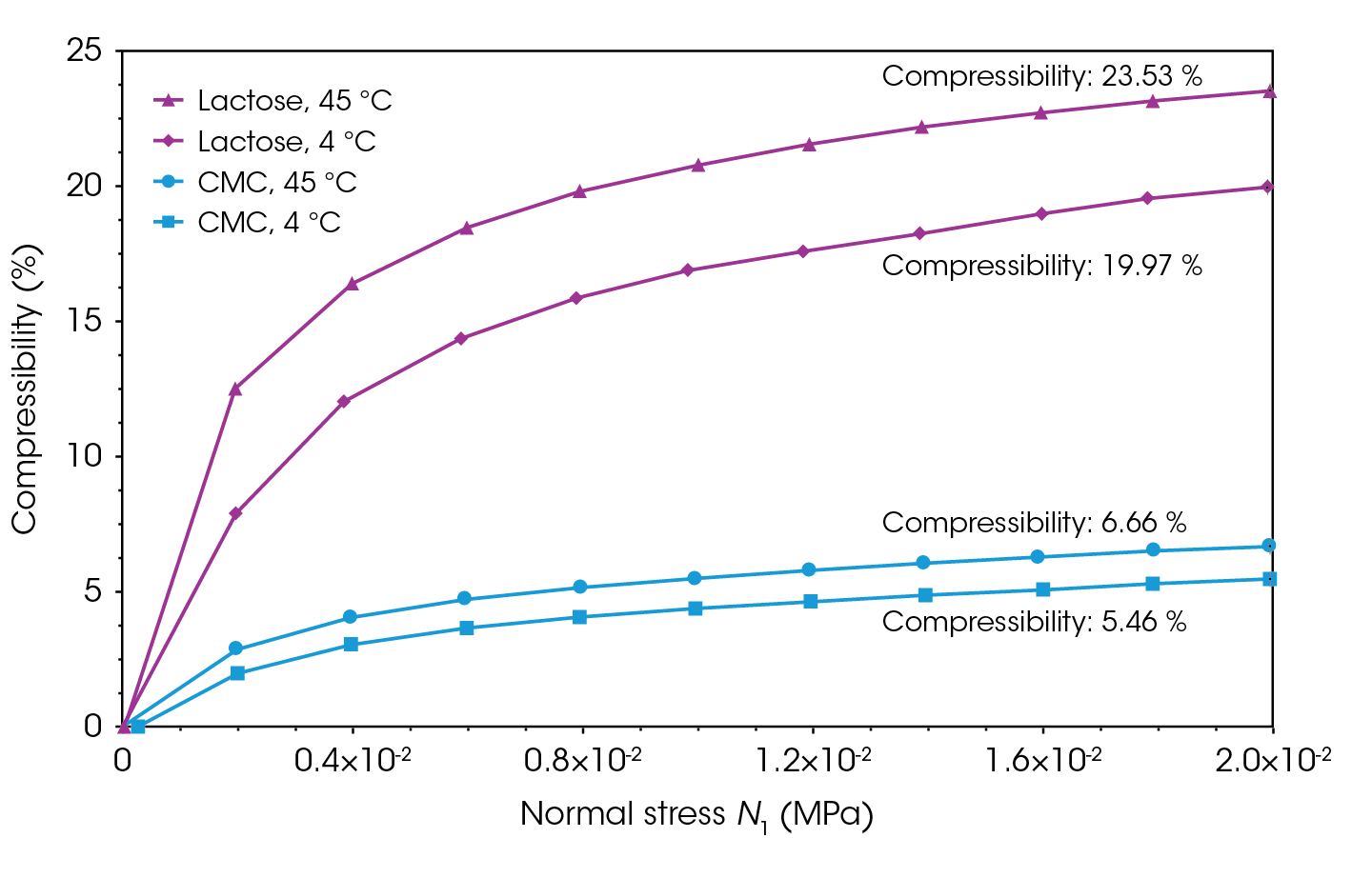
The compressibility of both powders were measured at distinct temperatures: 45 °C, mimicking stored under sunlight, and 4 °C, simulating refrigeration (see Figure 3 and Figure 4). Compressibility increased for both samples at 45 °C and decreased at 4 °C. Studies have shown that with less moisture at higher temperatures, lactose monohydrate becomes more brittle. Brittle materials tend to fracture more easily under pressure, which can sometimes enhance compressibility because the smaller fragments can pack more tightly together [2,11-13]. Similarly, CMC is hygroscopic, meaning it readily absorbs moisture from the environment. When temperature is increased, CMC loses some of its absorbed moisture, lowering the chance of particles sticking together and forming clumps, making the material easier to compress [2,11‑13].
Conversely, powders are more likely to gain humidity at 4 °C compared to room temperature due to lower evaporation rates and better moisture retention. High humidity can cause particles to stick together, and the resulting clumps are less able to rearrange and pack efficiently under pressure [11-13].
Excipient powders that compact well ensure that the blend can be easily compressed into solid tablet form. These powders provide the necessary bulk and mechanical strength to the tablet, making the manufacturing process more efficient and the final product more stable [4,7,8].
Flow function & cohesion
Figure 6 presents the shear results for lactose monohydrate. The tests were conducted in both standard and precision modes to assess the repeatability of the results (consolidation stress of 0.015 MPa). The precision mode produced a flow function of 5.23, which is slightly lower than the standard mode, but the values are in good agreement.
TRIOS™ Software was utilized to perform yield locus analysis and calculate results according to ASTM D7891. The steady-state stress of all pre-shear steps was determined and averaged. For each shear step, the yield point (the initial point at which a material begins to flow) was obtained and plotted.
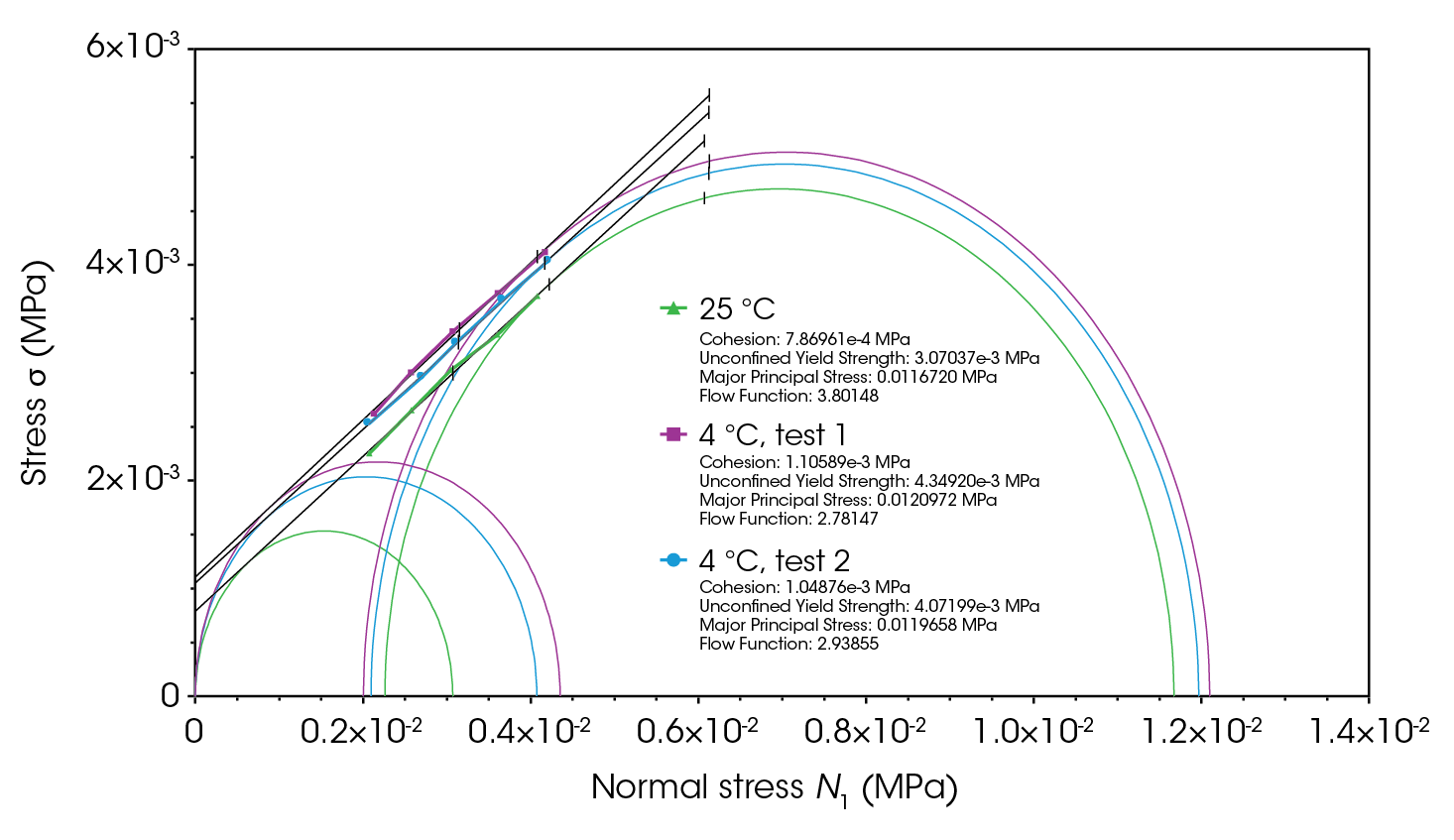
The yield locus line is a line of best fit drawn through the shear data points, with the y-intercept indicating the cohesion between particles. A “first” Mohr circle is drawn to pass through the graph origin and lie tangent to the yield locus line; its x-intercept represents the unconfined yield strength. A second Mohr circle is drawn to pass through the pre-shear average coordinates and lie tangent to the yield locus line. The greater x-intercept of this circle indicates the major principal stress (the maximum internal stress under consolidation) [1,3]. The flow function is the ratio of the major principal stress to the unconfined yield strength.
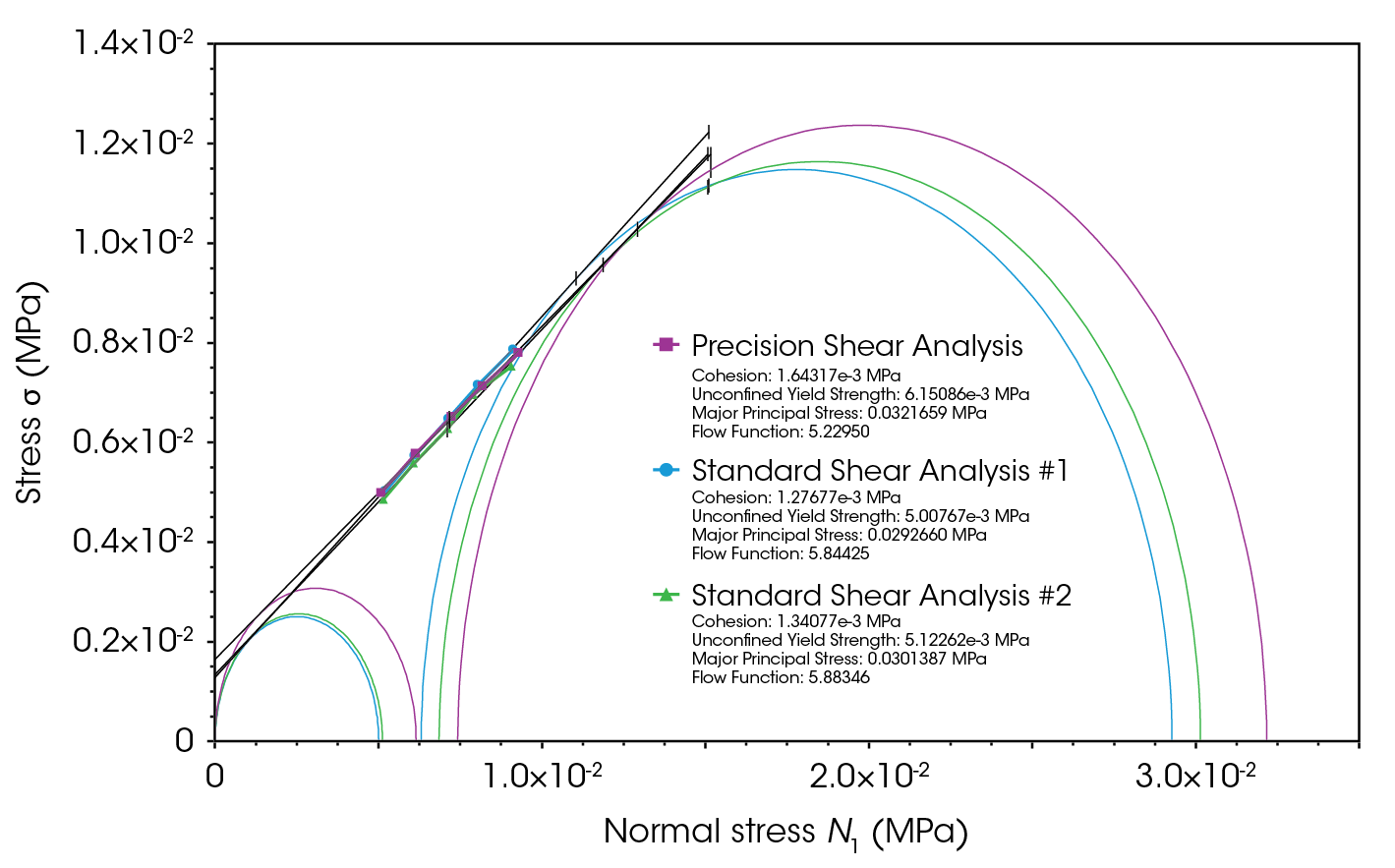
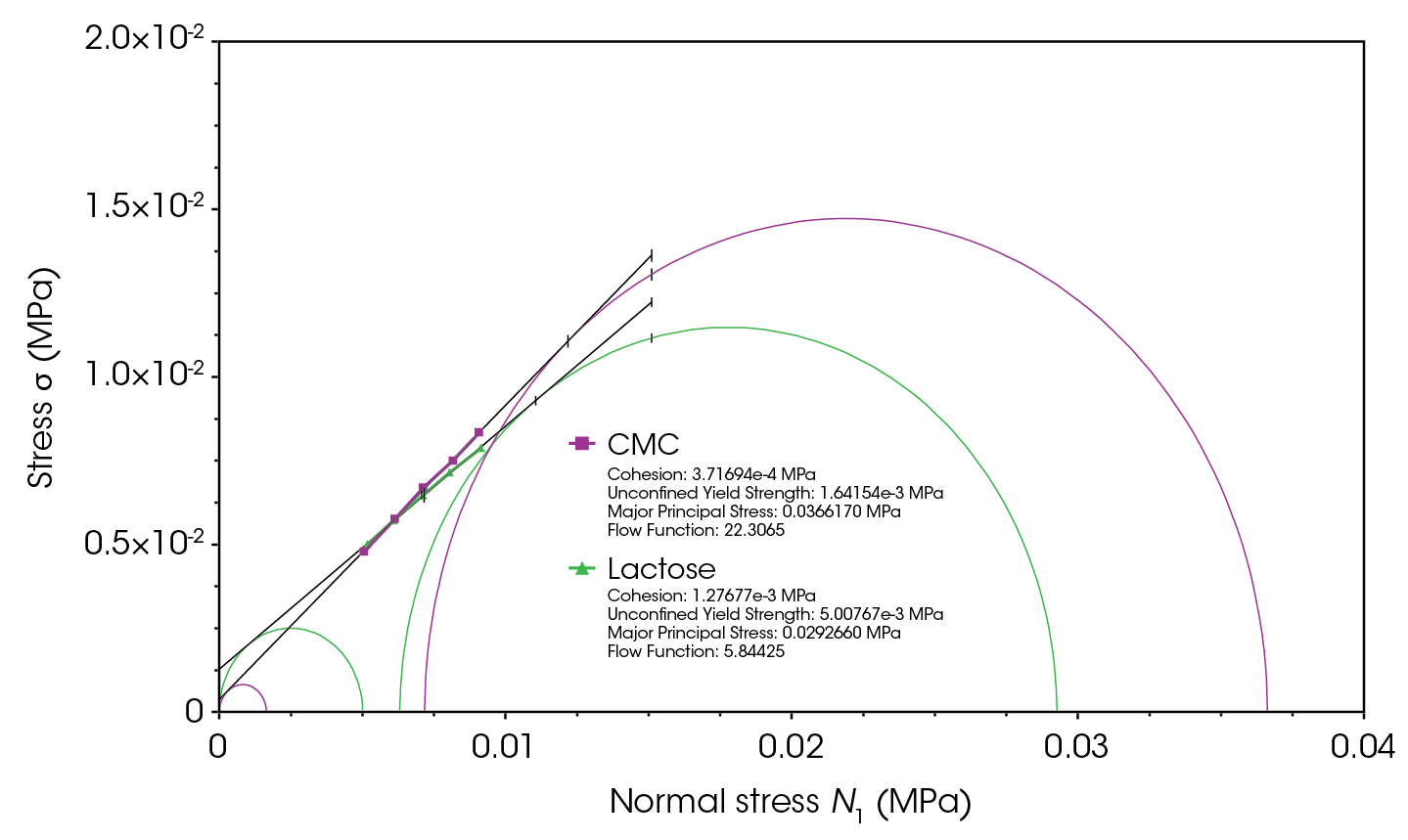
Figure 7 shows that lactose monohydrate exhibits three times higher cohesion than CMC. This is attributed to lactose having a higher percentage of finer particles, as observed in SEM images. Smaller particles have a larger surface area relative to their volume, leading to increased interparticle friction and cohesion. Cohesive forces significantly contribute to resistance to flow [4-6,10,11,13,14]. It is also observed that the flow function for CMC is almost four times higher than that of lactose monohydrate. Powders are categorized by their flow function values (ffc) into the following regions: free-flowing (ffc > 10), easy-flowing (4 < ffc ≤ 10), cohesive (2 < ffc ≤ 4), very cohesive (1 < ffc ≤ 2), and non-flowing (ffc ≤ 1) [15-17]. Thus, while lactose monohydrate falls within the easy-flowing region, CMC is categorized in the free-flowing region.
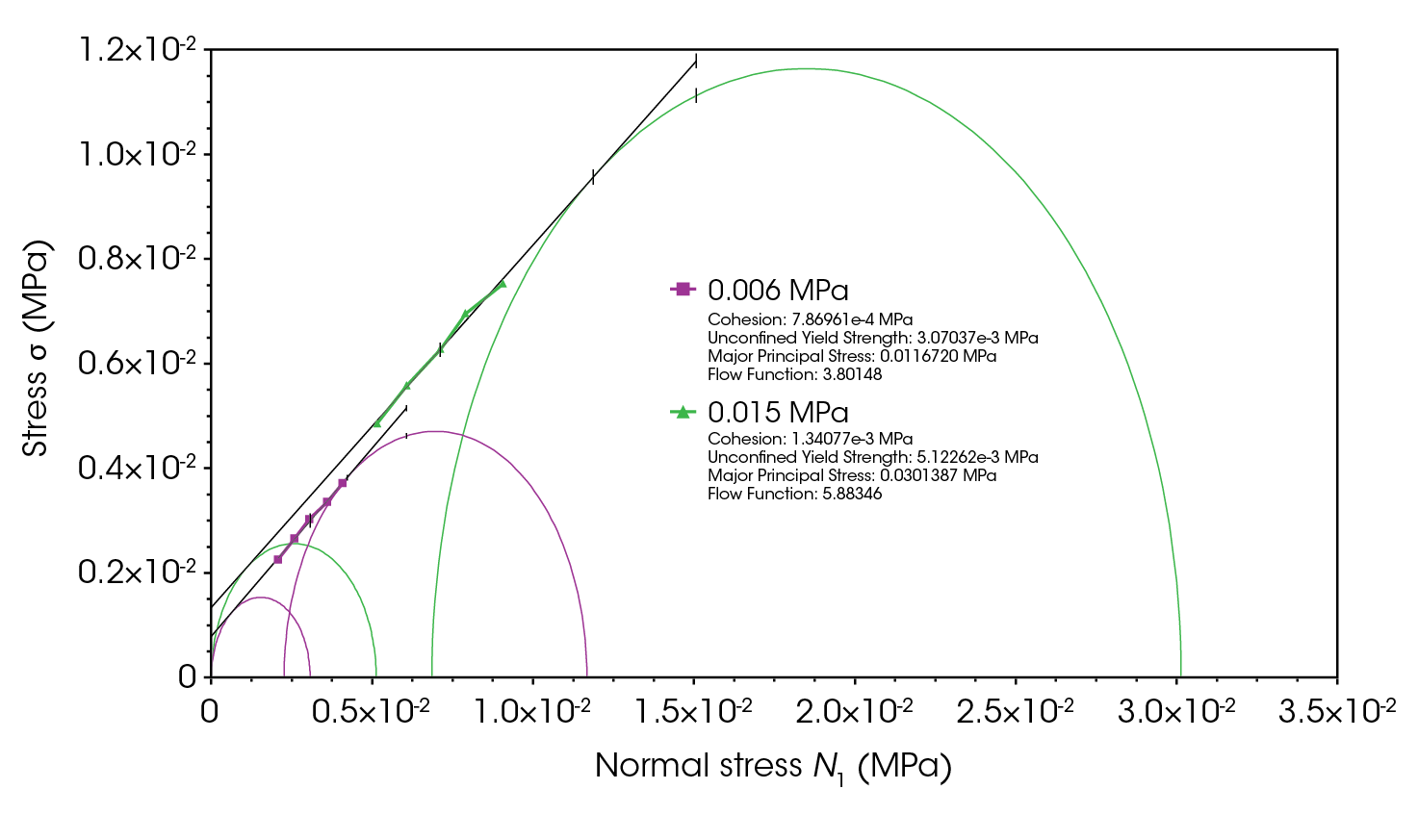
As lactose monohydrate exhibits higher compressibility, its flow function under lower consolidation stress is also evaluated (see Figure 8). When consolidation stress decreases, lactose’s ability to flow smoothly and consistently diminishes, reducing the flow function from 5.88 to 3.8. It appears reduced consolidation stress weakens the forces holding the particles together and increases void spaces between particles, disrupting uniform flow and leading to poor flow properties [5,6].
Confined and unconfined flow
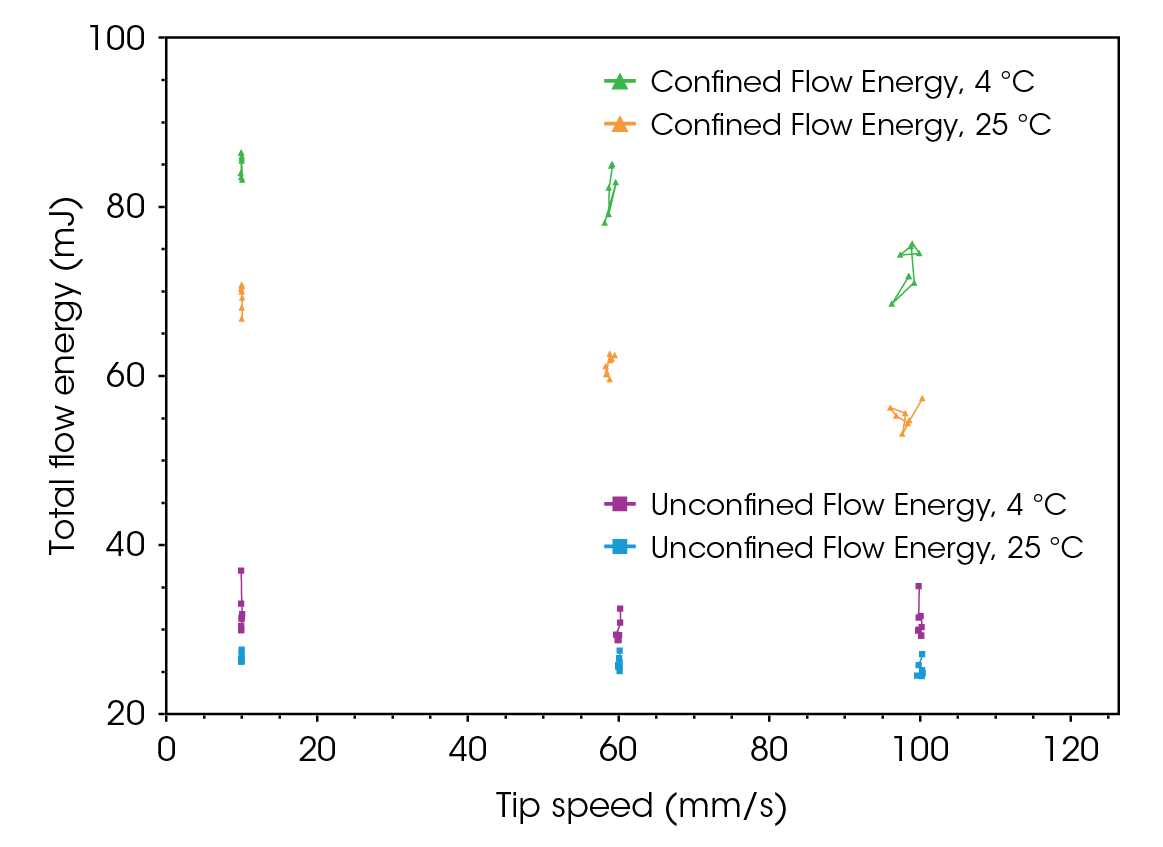
The flowability energy results for lactose monohydrate and CMC are illustrated in Figure 9 and Figure 10, respectively. Unlike the flow function, which indicates the flowability of powder under high stress, confined and unconfined flow energy values represent the flowability of powders under medium to zero external stresses. In die filling and tableting, unconfined flow energy helps predict how well the powder will flow into the die or mold. Confined flow energy measures the flow of a powder when it is under stress, such as during compression in a die. When the unconfined and confined flow energy is too high, it results in inadequate flowability, poor mixing, and inconsistent dosing. If the flow energy is too low, the material may flow too easily, leading to voids, segregation, and inconsistencies [7,9].
The confined flow energy at different tip speeds is, on average, 18% higher for CMC than lactose monohydrate. In contrast, the difference in unconfined flow energy is minimal.
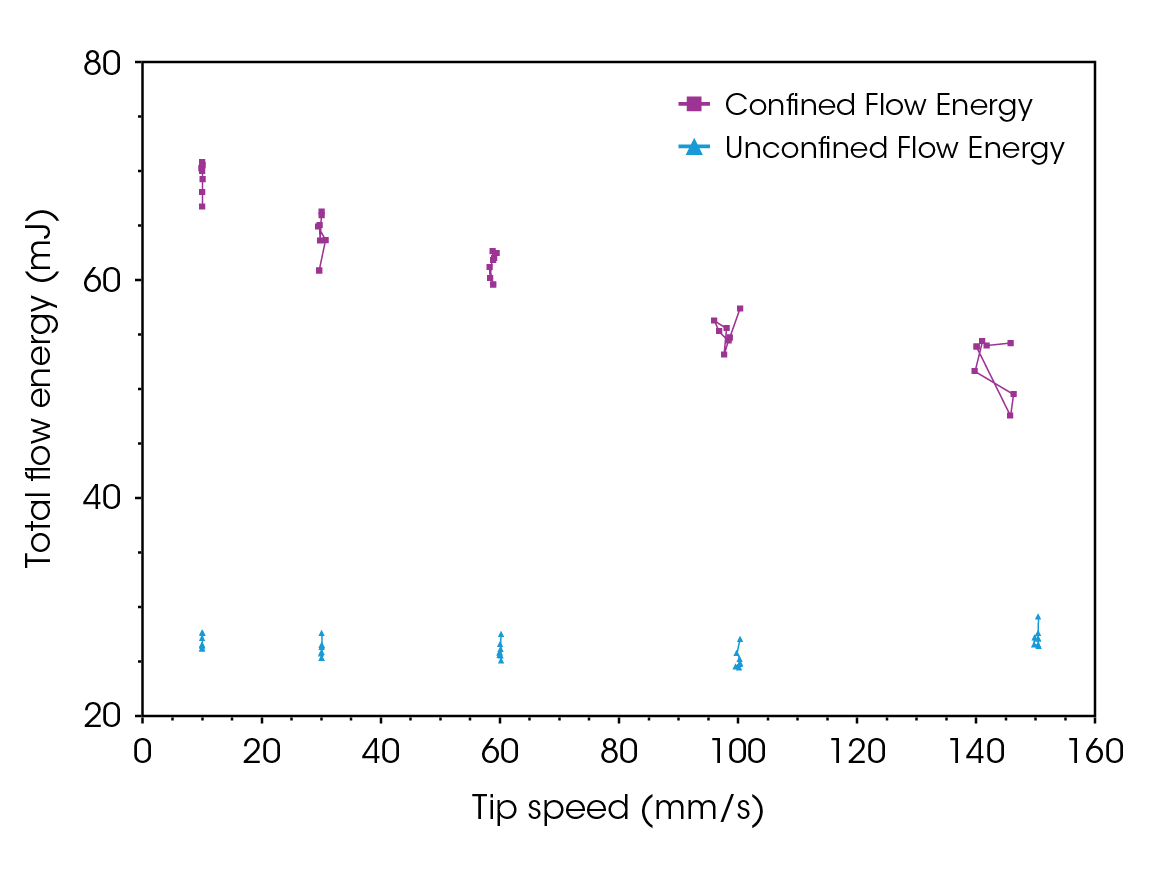
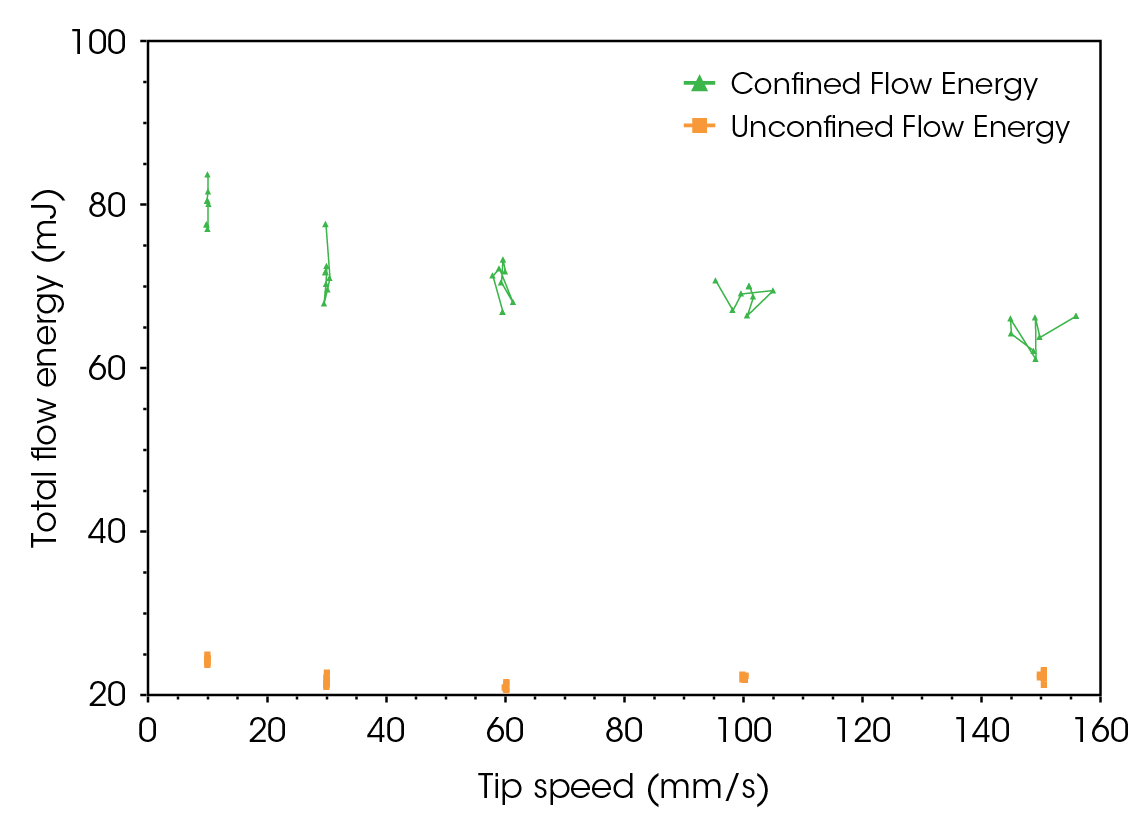
Both powders exhibit rate-dependency; the confined flow energy decreases as tip speed increases by 13% for lactose monohydrate and 11% for CMC. This phenomenon can be viewed as analogous to shear thinning observed in soft materials. Increasing the speed (tip speed for powders, shear rate for soft materials) leads to the alignment of particles or internal structures, reducing resistance to flow. Both phenomena result in easier flow under higher stress conditions. This could be both advantageous and disadvantageous, depending on the process. However, it is crucial information for formulators during early-stage drug development, as it could lead to significant differences in processability during scale-up.
Temperature effect
Figure 11 and Figure 12 illustrate the effect of higher temperatures on the flow function and cohesion of lactose monohydrate and CMC. Generally, higher temperatures enhance powder flowability by reducing humidity and interparticle cohesive forces, making it easier for particles to move past each other. However, a small amount of moisture can act as a lubricant, aiding particle movement [4,10,12-14].
Conversely, lower temperatures and higher humidity increase cohesive forces between particles, making the powder more prone to clumping and reducing its flowability [2,4,10,12-14].
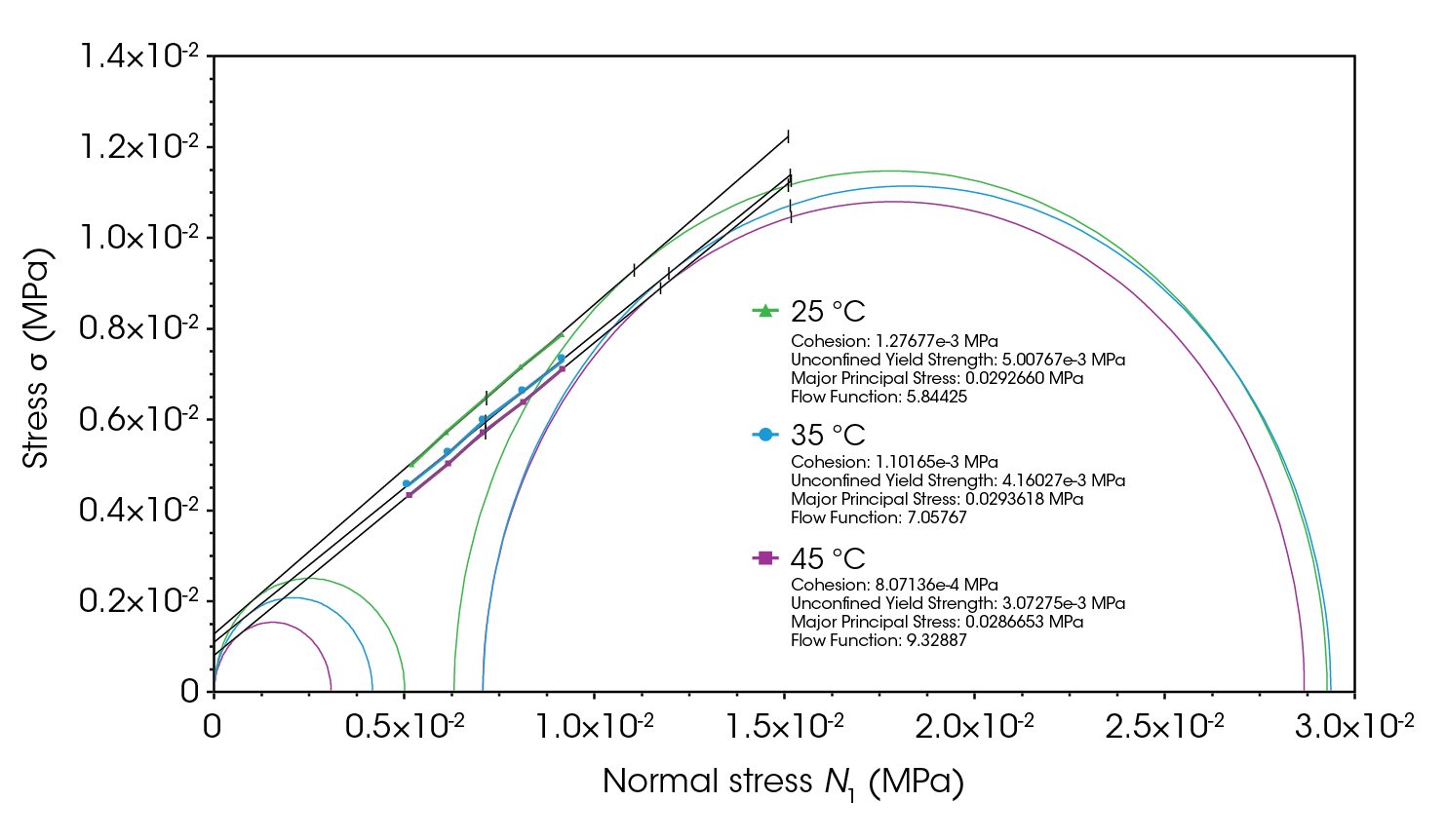
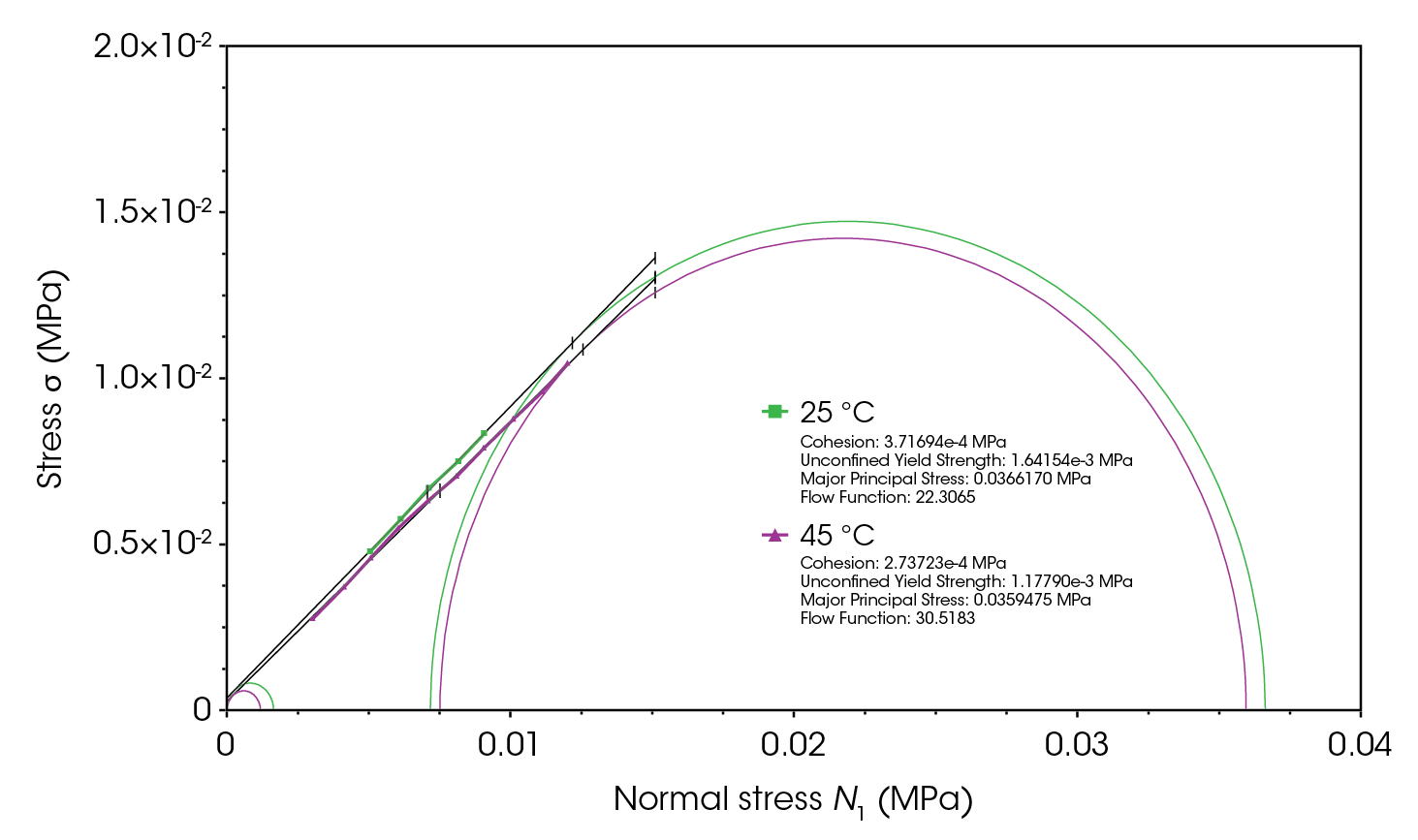
Since lactose is more cohesive than CMC, tests were conducted to examine the effect of refrigeration on its flowability and flow function. As shown in Figure 13, the confined flow energy for lactose at 4 °C increased by an average of 29%, and the unconfined flow energy increased by 20% compared to the respective values at room temperature. This increase is again a consequence of higher moisture at lower temperatures, which enhances cohesion [4,10,12,13].
For cohesive powders, moisture can lead to the formation of liquid bridges between particles, increasing interparticle cohesive forces and significantly hindering flowability. Figure 14 shows that the flow function of lactose at 4 °C has shifted towards cohesive flow region (consolidation stress of 0.006 MPa), which is undesirable for a filler excipient. The shear test at 4 °C was repeated twice in both standard and precision modes to ensure data accuracy, with the slightly lower flow function resulting from the precision method [4,10,13,14].
Conclusions
CMC and lactose monohydrate are commonly used excipients in the pharmaceutical industry. By performing measurements of dynamic flowability over a range of processing speeds, it was found that both excipients exhibited a rate dependent flow energy. The confined flow energy decreases by 13% for lactose and 11% for CMC as the speed increases from 10 mm/s to 150 mm/s. The confined flow energy at different tip speeds is, on average, 18% higher for CMC. However, the difference in unconfined flow energy is minimal.
Lactose monohydrate exhibited over three times higher compression and cohesion values compared to CMC. This finding aligns with the fact that the flow function for lactose falls in the easy-flowing region, while CMC is in the free-flowing region.
To assess thermal sensitivity, powder rheology properties were examined at 45 °C simulating exposure to sunlight and at 4 °C simulating refrigeration. At 4 °C, lactose monohydrate’s confined flow energy increased by 29%, unconfined flow energy by 20%, and the flow function shifted towards cohesive flow region. At 45 °C, the flow function of both excipients increased, likely due to lower humidity levels and reduced cohesion. Additionally, compressibility of both excipients increased at 45 °C and decreased at 4 °C, consistent with the observed role of humidity.
Lactose monohydrate shows easy flow, relatively low confined flow energy, moderate cohesion, and excellent compressibility. CMC, however, has lower cohesion but higher confined flow energy. These results can aid formulators and researchers in optimizing fillers and binders, as well as processing of their products. Additionally, while not explored in this work, powder rheology could aid in finding the right proportions of CMC and lactose combinations to create a more effective binder and filler.
References
- TA instruments, “RH123: Powder Rheology of Lactose,” 2022. [Online]. Available: https://www.tainstruments.com/pdf/literature/RH123.pdf.
- TA instruments, “RH126: Effect of Moisture on Cohesion Strength of Carboxymethyl Cellulose Powder,” 2023. [Online]. Available: https://www.tainstruments.com/pdf/literature/RH126.pdf.
- S. Cotts and M. Ulrich, “Powder Rheology for Pharmaceutical Development,” TA Instruments, New castle, 2023.
- R. Suhag, A. Kellil and M. Razem, “Factors Influencing Food Powder Flowability,” Powders, vol. 3, no. 1, pp. 65-76, 2024.
- L. Y. Leung, C. Mao, I. Srivastava, P. Du and C. Y. Yang, “Flow function of pharmaceutical powders is predominantly governed by cohesion, not by friction coefficients,” Journal of pharmaceutical sciences, vol. 106, no. 7, pp. 1865-1873, 2017.
- Z. X., N. E., N. A. and M. C., “Flow Function of Pharmaceutical Powders at Low-Stress Conditions Can Be Inferred Using a Simple Flow-Through-Orifice Device,” Journal of Pharmaceutical Sciences, vol. 109, no. 6, pp. 2009-2017, 2020.
- R. Sedlock, “Powder die filling: An essential process for quality tablets,” Manufacturing Chemist., 30 November 2020. [Online]. Available: https://www.manufacturingchemist.com/powderdie-filling-an-essential-process-for-quality-tablets-172198. [Accessed August 2024].
- D. S. Shah, K. K. Moravkar, D. K. Jha , V. Lonkar, P. D. Amin and . S. S. Chalikwar, “A concise summary of powder processing methodologies for flow enhancement,” Heliyon, vol. 9, no. 6, p. e16498, 2023.
- P. H. M. Janssen, S. Depaifve, A. Neveu , F. Francqui and B. H. J. Dickhoff, “Impact of Powder Properties on the Rheological Behavior of Excipients,” Pharmaceutics, vol. 13, no. 8, p. 1198, 2021.
- D. Bourcier, J. P. Féraud, D. Colson, K. Mandrick, D. Ode, E. Brackx and F. Puel, “Influence of particle size and shape properties on cake resistance and compressibility during pressure filtration,” Chemical Engineering Science, vol. 144, pp. 176-187, 2016.
- A. Crouter and L. Briens, “The effect of moisture on the flowability of pharmaceutical excipients,” Aaps PharmSciTech, vol. 15, pp. 65-74, 2014.
- Covestro, “Applications and Properties of Sodium Carboxymethyl Cellulose,” AZoM, 30 October 2019. [Online]. Available: https://www.azom.com/article.aspx?ArticleID=2788.. [Accessed 02 August 2024].
- X. Y. Lu, L. Chen, C. Y. Wu, H. K. Chan and T. Freeman, “The Effects of Relative Humidity on the Flowability and Dispersion Performance of Lactose Mixtures,” Materials (Basel), vol. 10, no. 6, p. E592, 2017.
- M. Stankovic-Brandl, S. Zellnitz, P. Wirnsberger, M. Kobler and A. Paudel, “The influence of relative humidity and storage conditions on the physico-chemical properties of inhalation grade fine lactose,” AAPS PharmSciTech, vol. 23, pp. 1-13, 2022.
- M. Mariano, “General aspects of powder rheology applied to pharmaceutical formulations,” Drug Discovery Today, vol. 29, no. 5, 2024.
- D. Schulze, Powders and bulk solids, Berlin/Heidelberg: Germany: Springer International Publishing, 2021.
- D. M. Parikh, “Handbook of pharmaceutical granulation technology,” Drugs and the pharmaceutical sciences, vol. 81, 2005.
Acknowledgement
For more information or to request a product quote, please visit www.tainstruments.com to locate your local sales office information.
This paper was written by Behbood Abedi, PhD.
TA Instruments, TRIOS, and Discovery are trademarks of Waters Technologies Corporation.
Click here to download the printable version of this application note.

