Keywords: ITC, affinity, enthalpy, entropy, binding
MC165
Introduction
Isothermal titration calorimetry is a technique where heat is measured under constant pressure. This heat can be translated into enthalpy based on the First Law of Thermodynamics. Although the focus of the technique has been rooted in measuring biophysical phenomena in labs that specialized in calorimetry, it has seen growth as a general technique to quantitatively describe systems from proteins to nanomaterials. This affinity assay is a native test that doesn’t rely on intrinsic or extrinsic fluorphores like other binding techniques, and neither of the binding partners are immobilized onto a chip or probe. Additionally, ITC provides more than affinity; all the thermodynamic properties as well as stoichiometry of chemical or physical equilibria are measured in a single experiment.
“Rapid Assay and Analysis”
One critique of ITC is that it requires significant amounts of material and time. It is true that errors in the reported fitting parameters decrease when working at a cell concentration between 1 to 1000 times the Kd and experimental time is from one to two hours. However, these are the old rules from close to three decades ago where the full incremental titration profile was the ideal and peak resolution had not been optimized. Under these rules it could be difficult to consider ITC for high through-put screening (HTS). Typically, HTS is done solely for the IC50 value or Kd. However, it has been shown that the compound with the highest Kd value is not always the compound with the highest potency (Rykmans). This honor is given to those compounds with the largest difference between the logKd and hydrophobicity, coined the “LipE value” (Rykmans). More simply stated, LipE removes the entropic (hydrophobic) component of binding. In separate evaluations, it has been determined that the LipE value is proportional to the enthalpy and compounds with the most favorable enthalpy also have the most favorable LipE value(Ross). The new ideology is “how can you afford not to screen for enthalpy?”
If the goal is HTS, this can be completed with minimal loss of titrant in the syringe/titrant, which is typically the protein or DNA target. Non-saturating conditions are recommended where the majority of the titrant will bind to the titrand. The heat generated from each injection is normalized to the titrant and can be advantageous as there are many situations where the concentration uncertainty is greater for the library of compounds.
ITCRUN™ software and the Affinity ITC were designed with screening in mind. The 250 μL injection syringe of the LV Affinity ITC enables screening of more than 20 compounds with a single syringe load and the standard 3 injection x 3 μL assay.
For more information on how perform HTS on ITC, refer to the application notes MCAPN-2016-02, MCAPN-2013-03 from TA Instruments and the Analytical Biochemistry reference from Schön and Freire.
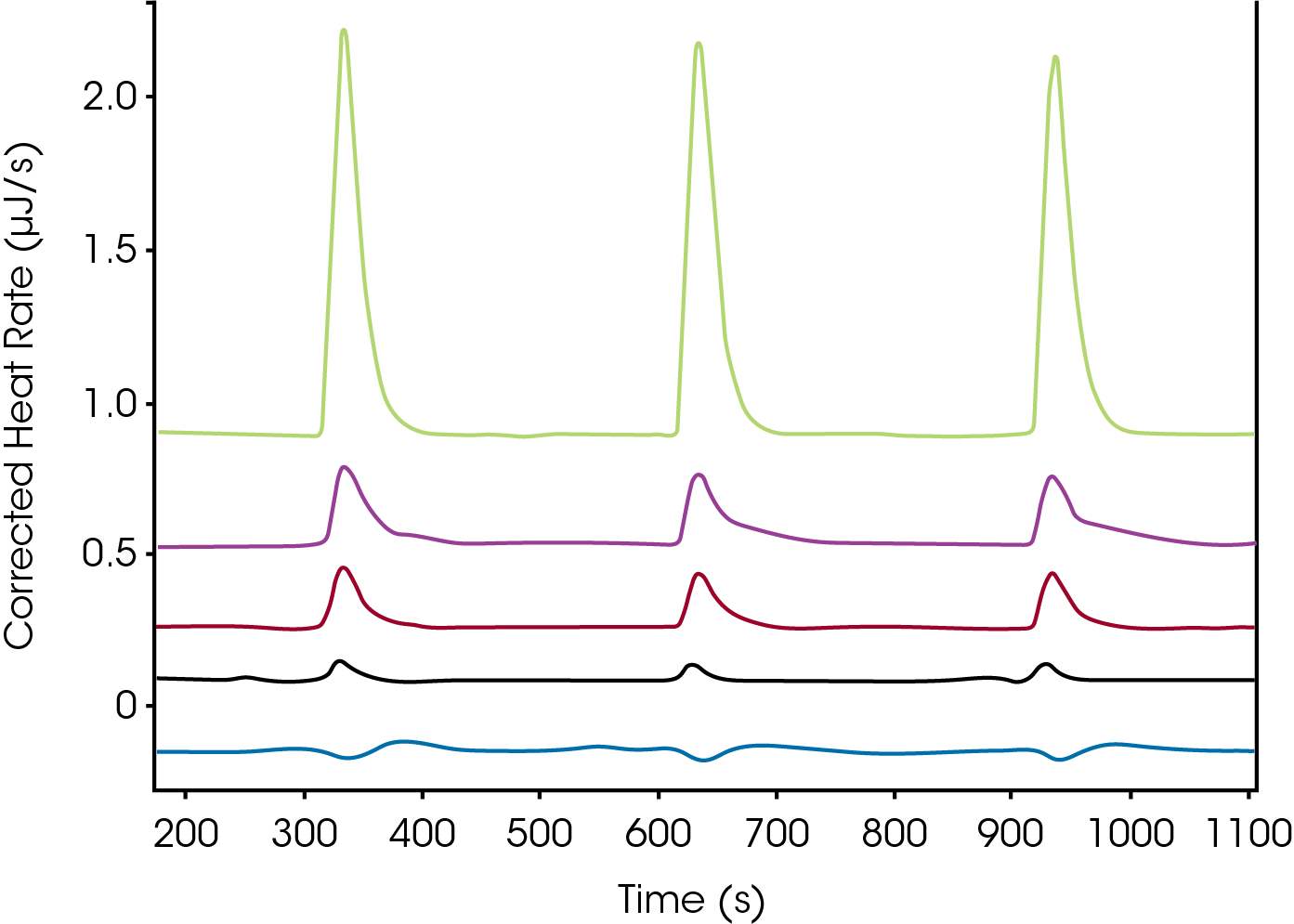
After screening (Figure 2), the top hits are investigated through a full titration in pursuit of an accurate Kd and n.
The importance of enthalpy is not isolated to pharmaceutical applications. This thermodynamic parameter defines the binding and how the reaction has changed a chemical system. Enthalpy describes the specific interactions such as hydrogen bonding, proton coupled interactions and electrostatic interactions. This idea is nicely illustrated by Ladbury et al, where interpretation of the enthalpy described the effect of a water molecule in the binding pocket for one ligand and absent from the pocket for another ligand. (Ladbury, Klebe, Freire).
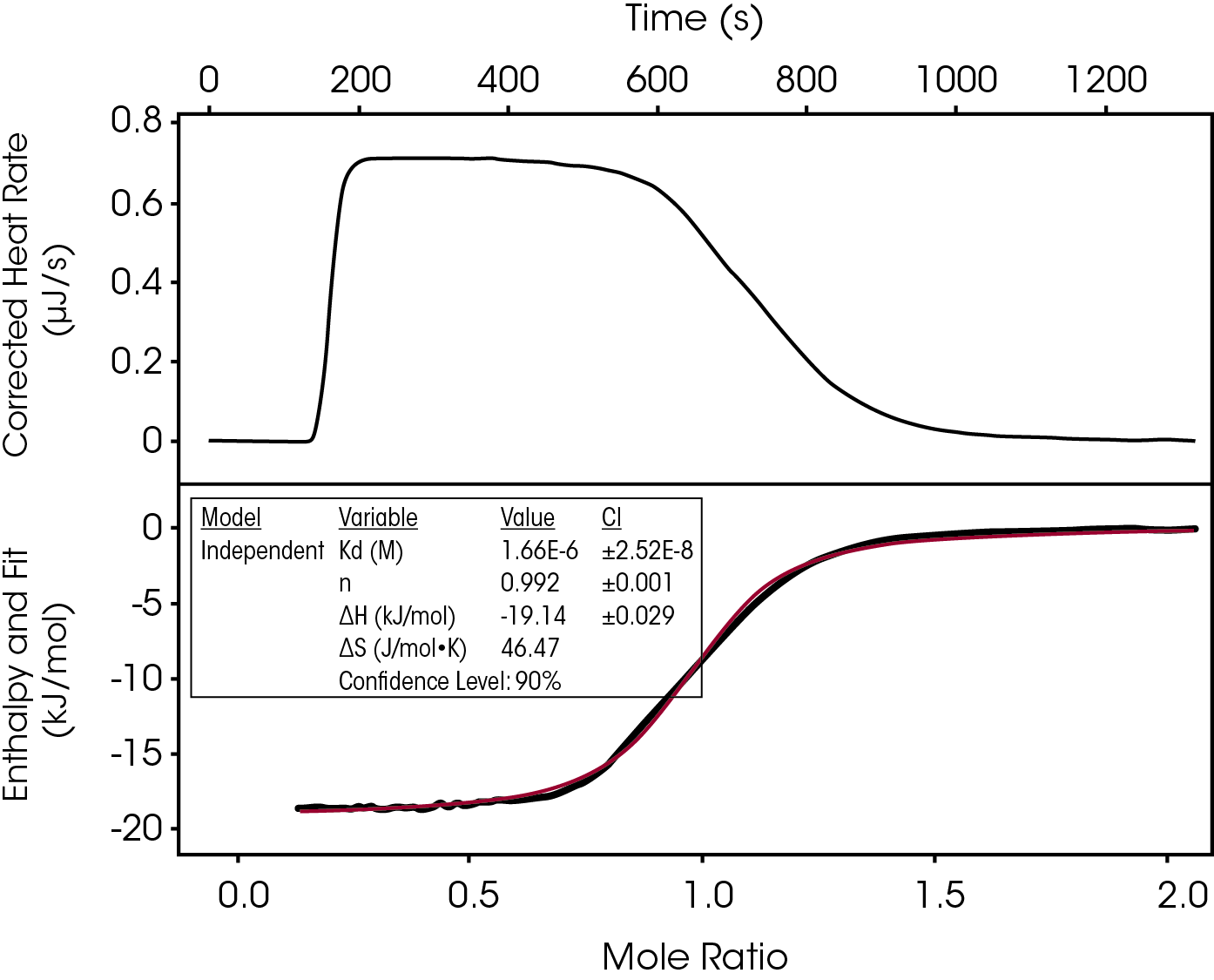
Another fast binding experiment and ITC technique that has started to gain popularity is the continuous titration. By slowly delivering the titrant under standard 10:1 titrant:trand conditions the full thermodynamic profile is captured in about 20 minutes (Figure 3).
Baseline Determination
Baseline designation can be one of the more subjective processes applied to ITC data. NanoAnalyze® provides users a tool to remove subjectivity with the “AUTO” baseline application. Baseline determination on the Affinity ITC was also improved with more efficient mixing thereby decreasing peak spread. Efficient mixing and ideal introduction of the titrant into the titrand also reduces the need for a filter. Mixing efficiency enables users to collect rapid baseline resolved data without the addition of a mathematical filter. Mathematical filters applied to data in order to create a sharp peak can make time constant determination inaccurate along with the possibility of introducing errors in the other thermodynamic values. A filter like this would lead a user to believe that the time constant was smaller than reported specifications and can lead to inaccuracies in reported kinetic values.
Baseline Stability
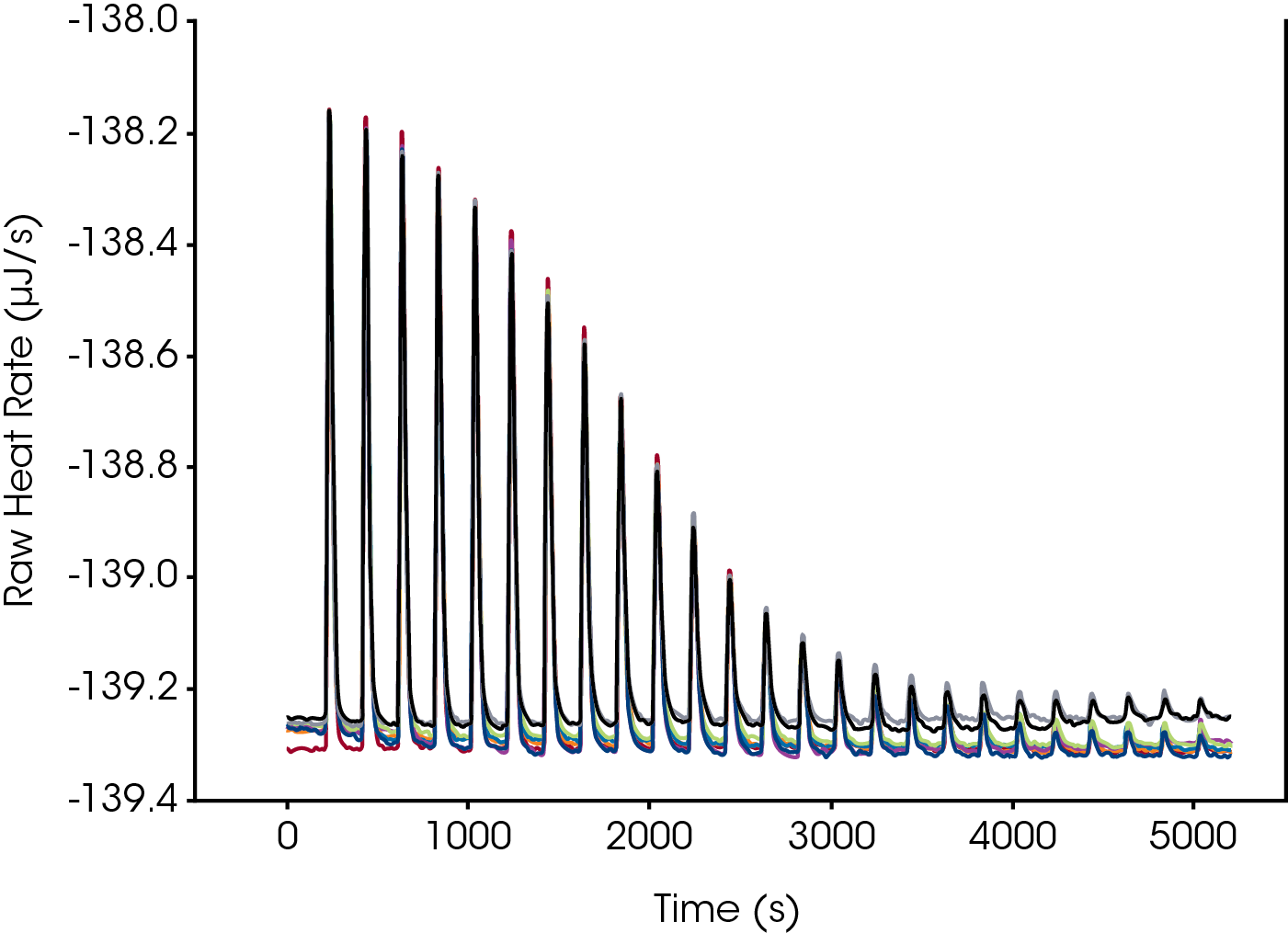
What does it mean to not have a slope in the raw data? In a power compensation instrument, the calorimeter measurement cell is controlled at a constant temperature. This is accomplished by means of applying constant cooling to the cell and then using a temperature controller and heater to keep the cell temperature constant. The raw signal in the power compensation calorimeter is the power (mcal/sec or mJ/sec) applied to the control heater that is required to keep the calorimeter cell from changing temperature as a function of time. Not all calorimeters perform in this way and one can typically tell that passive control is used by a slope in the raw data. With automated delivery of titrand not only is the slope stable due to instrument control, but the offset from one run to the next is minimal Figure 4 indicating precision in loading the sample and control of the instrument over the course of a day all while maintaining a constant temperature.
A stable baseline makes peak determination easier and has a substantial role in kinetic experiments that directly uses dQ/dt, the baseline, in Km and Vmax determination. It could also open the application field for this calorimeter to include isothermal experiments that depend on baseline precision.
Reproducibility and Reliability
TA Instruments uses a trusted Spark Holland autosampler. The fully automated systems allow the user to select up to six solutions for “tip to “tip” cleaning of the inside and outside of parts that will encounter the titrant and titrand. From the autosampler the Affinity system takes over the liquid handling and moving parts are minimized. For the semi-automated instrument six solutions can also be selected for automated cleaning of the inside and outside of the titration syringe.
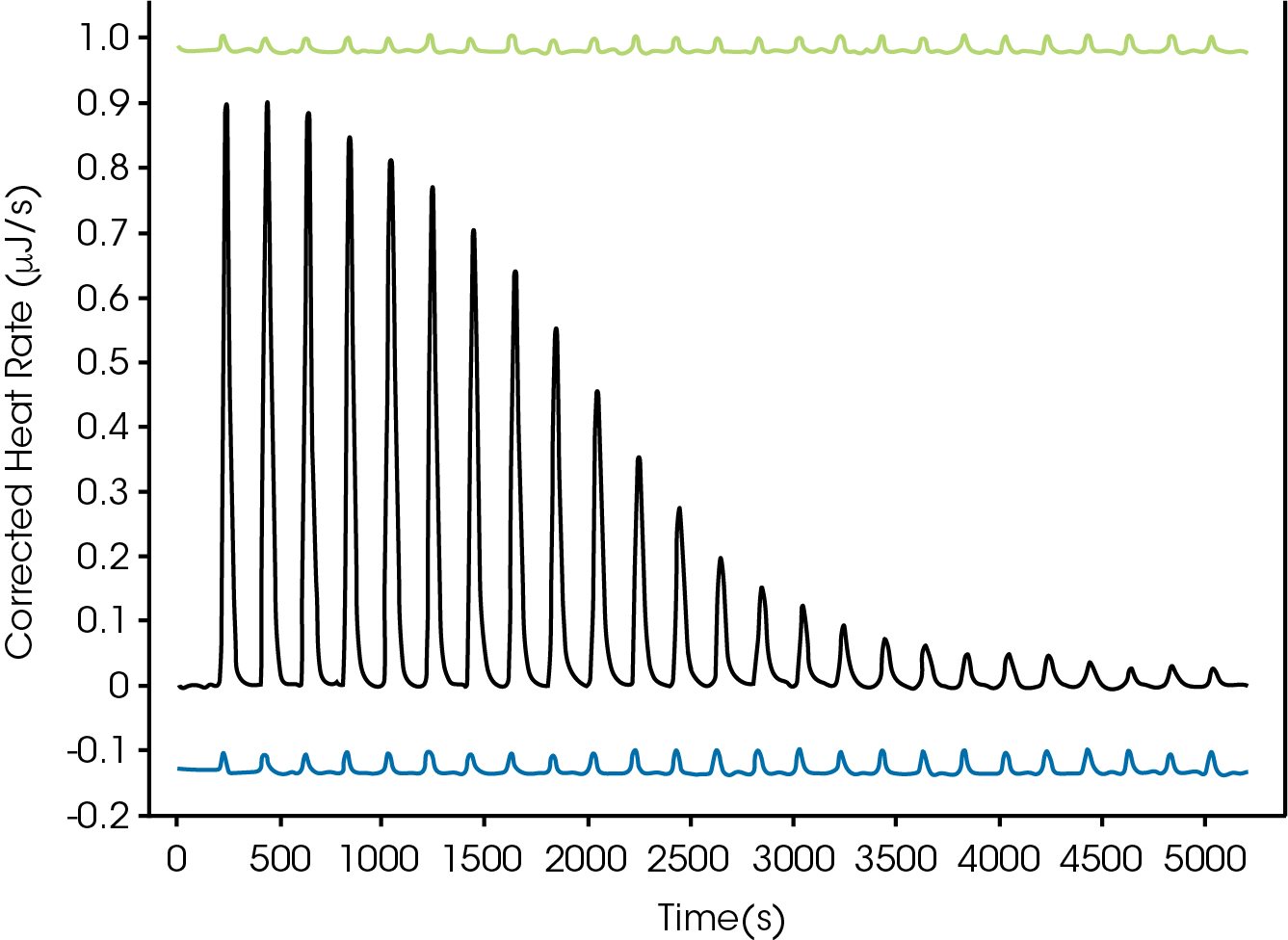
Cleaning is an essential step to collect quality and accurate data. To demonstrate if the syringe is cleaned a buffer-buffer or water-water experiment can be collected before and after the titration (Figure 5).
If there was residual titrant in the syringe or cell the buffer or water titrations would show either a large or inconsistent dilution heat. The fully automated Affinity has six different solutions to clean the autosampler, cell, and syringe. The semi-automated instrument has a valve that will accommodate six solutions for automated cleaning of the titration syringe.
Delivery and Mixing
For close to three decades the major commercial manufacturers for ITC instruments have paired the delivery syringe and stirring mechanism. Separating these two components turned out to be a breakthrough for mixing efficiency at low stir speeds. The former delivery mechanism older style can require higher stir rates that can damage samples.
With this new design fear of shearing DNA or “foaming” of proteins in the cells is avoided. The geometry of the cell and directed delivery led to efficient mixing at stir rates between 75 to 125 rpm – 10x slower than rates reported in the literature!
The titration of sucrose into water, a standard proposed in an IUPAC document (Wadso and Goldberg) can be accurately measured. The new system can mix solutions where the viscosity differences between two solutions is substantial. In addition to this efficient mixing the stirrer can turn in both a forward and reverse direction. In the reverse direction, the principle is that materials that settle more easily, such as nanomaterials and whole cells, will be pulled-up from the bottom and then in solution to be mixed with the titrant.

Thermodyamic Evaluation
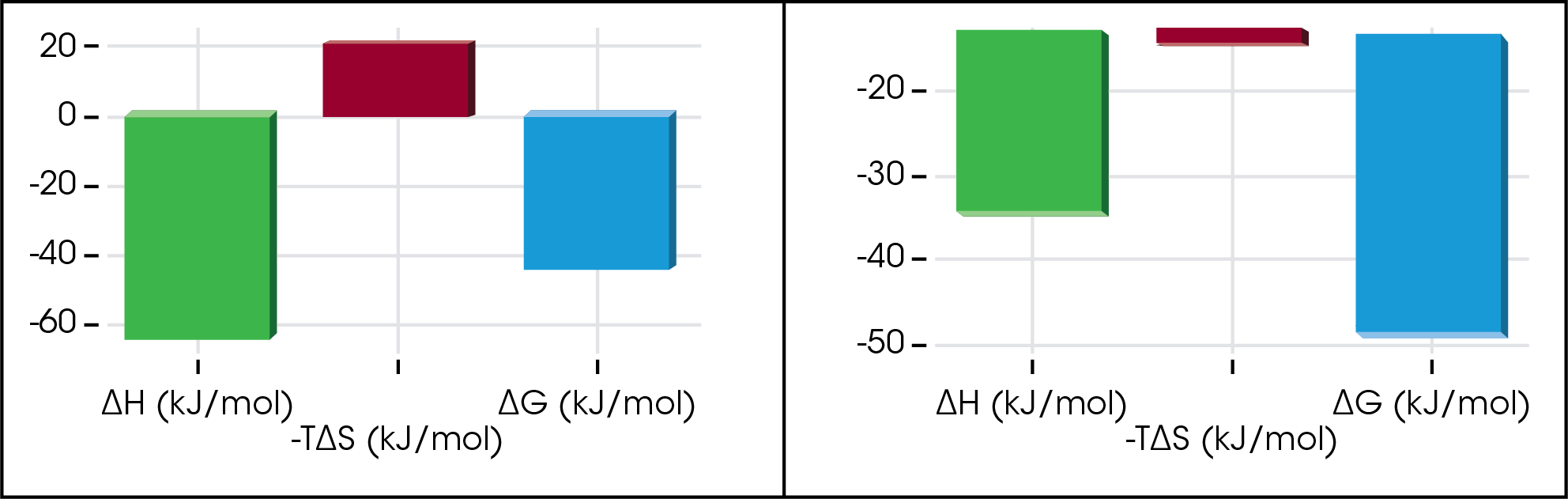
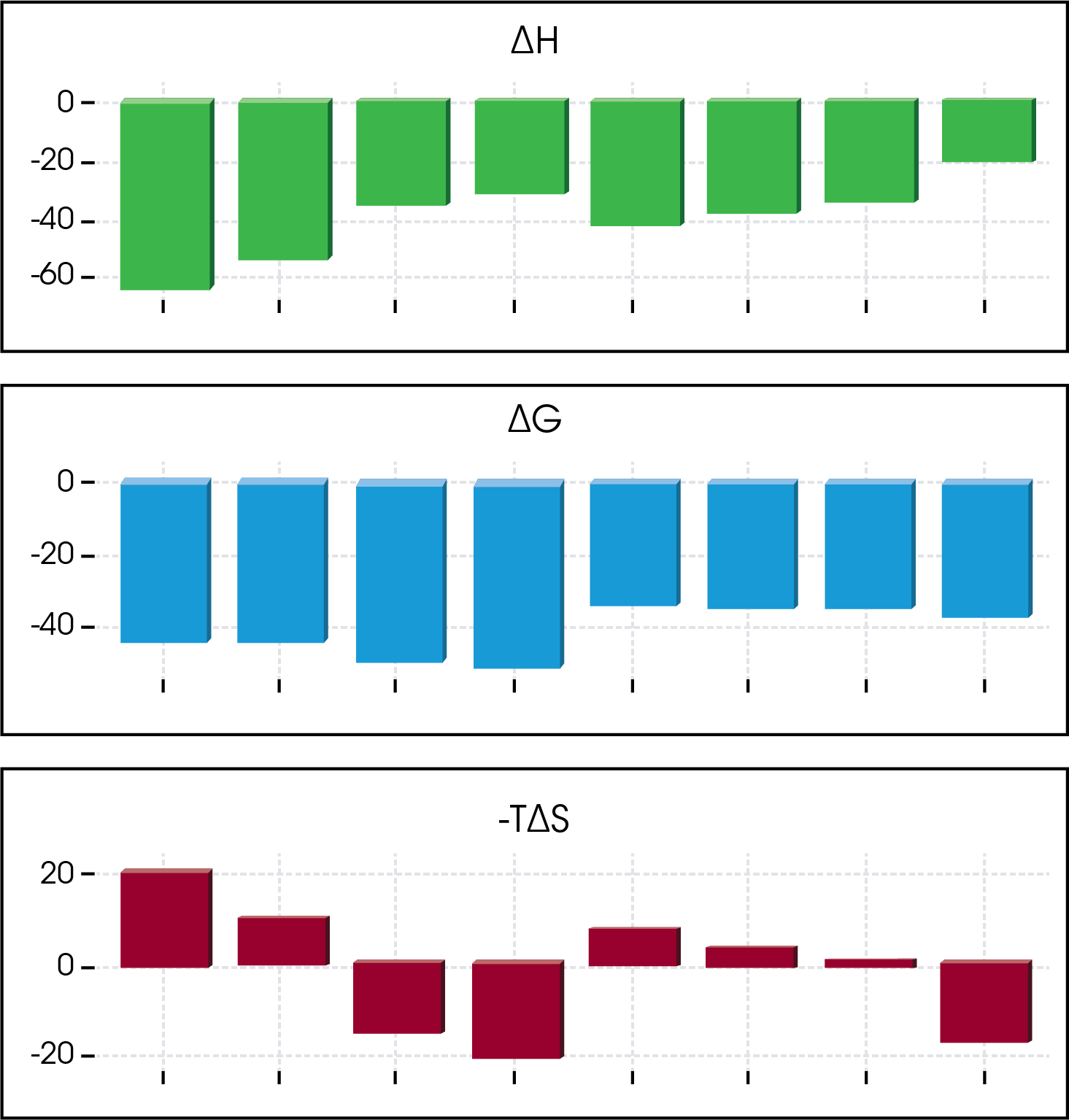
Tables of numbers can be overwhelming, but necessary. As an alternative, a quick view of the thermodynamic profiles can provide a full story by a glance.
Furthermore, these table values can be weighted based on the researchers’ preference. In the figure below a weighting factor of 5 was applied to the enthalpy and 2 was applied to the free energy and then the data was sorted. From this set, an interesting enthalpy-entropy compensation is discovered.
Summary
The advances mentioned above will enable investigators to expand their research using ITC. Higher throughput and intelligent data analysis will assist those in discovery and optimization. Advance stirring opens the discipline to solutions that were avoided because of poor mixing and shearing concerns. Kinetic endeavors using the technique will be improved because of the stable baseline. The stable baseline and excellent reproducibility helps with those that have small heats upon binding. Go Discover!
References
- Ross, P. and Freire, E. MCAPN-2013-03 “Enthalpy Screening by Isothermal Titration Calorimetry” available at www. tainstruments.com
- Ryckmans T, Edwards MP, Horne VA, Correia AM, Owen DR, Thompson LR, Tran I, Tutt MF, Young T “Rapid assessment of a novel series of selective CB(2) agonists using parallel synthesis protocols: A Lipophilic Efficiency (LipE) analysis”. Bioorg. Med. Chem. Lett. (2009) 19 (15): 4406–9.
- Frictional heat reference: “The art of calorimetry” Hansen L. and Hart, R. Thermochimica Acta (2004) 417:257-273
- Wadso, I. and Goldberg, R. “Standards in Isothermal Microcalorimetry” Pure Appl. Chem. (2001) 73(10):1625-1639, 2001
- Ladbury, J., Klebe, G., and Freire, E. “Adding Calorimetric Data to Decision Making in Lead Discovery: A Hot Tip” Nature Reviews. (2010) 9:23-27
- Schön, A. and Freire, E. MCAPN-2016-02 “Enthalpy Screen of Drug Candidates” available at www.tainstruments.com
- Schön, A. and Freire, E. “Enthalpy Screen of Drug Candidates” Analytical Biochemistry (2016) 513: 1-6
Click here to download the printable version of this application note.

