Keywords: Discovery SA, hygroscopicity, crystallinity, hydrates, pharma, ibuprofen, lactose
TA488
Abstract
Depending on the hygroscopic tendencies of the drug under investigation, exposure to water vapor (humidity) during production or storage can have a strong influence on its physical properties. Moisture adsorption can negatively affect a drug in many ways that could influence its stability, shelf-life, safety, efficacy, or bioavailability. This work presents three case studies to demonstrate the impact of moisture on the structural integrity of two active pharmaceutical ingredients: ibuprofen and sodium naproxen, along with a commonly used excipient, lactose.
Introduction
Effect of Moisture on Active Pharmaceutical Ingredients
The introduction of water can have highly consequential effects on the structure and morphology of active pharmaceutical ingredients (APIs). The propensity of a material to adsorb moisture reflects its hygroscopicity, which can impact the stability, efficacy, and dosing properties not only for the drug under investigation, but also for excipients, fillers, and polymeric delivery vehicles that go into the pharmaceutical product. Given the ubiquitous presence of water in the atmosphere and the central role it plays in human physiology, it is essential to understand a drug’s tendency to adsorb or desorb water in a controlled environment. This is achievable using the TA Instruments™ Discovery™ Sorption Analyzer (SA).
Aspirin served as an early case study for assessing the adverse effects of moisture on drug stability when, in 1958, Leeson and Mattocks proposed that moisture sorption led ultimately to decomposition by acid-catalyzed hydrolysis (1,2). Subsequent moisture sorption studies revealed that crystalline drug substances are generally less hygroscopic than their amorphous counterparts. Moreover, moisture sorption can profoundly affect the glass transition temperature (Tg) of amorphous polymers used commonly as API excipients. The Discovery SA provides a full characterization of the water sorption properties of a pharmaceutical, which can lead to valuable correlation between its stability, efficacy, and bioavailability.
The Discovery Sorption Analyzer
The Discovery SA shown in Figure 1 offers enhanced Dynamic Vapor Sorption (DVS) analysis by utilizing the Tru-Mass™ Balance with precise temperature and environmental control. Users can select a range of test profiles to obtain a deep understanding of the sorption properties of the material over a broad range of temperatures. Researchers have the option to program isothermal experiments while adjusting relative humidity (RH) in three ways: a continuous ramp, stepwise manner, or maintaining constant humidity (isohume) while adjusting the temperature in a stepwise or continuous manner. TRIOS™ Software is used in both the preand post-experiment workflow and offers seamless data continuity with the rest of the Discovery Suite available from TA Instruments.

Experimental
Ibuprofen
Ibuprofen powder (3.5 mg) was loaded into a metalized quartz pan and equilibrated at 25.0 °C. Humidity was set to 0% and, following an isothermal hold, humidity was stepped in increments of 10% RH to 90.0% RH. The desorption profile included stepping humidity in increments of 10% RH from 90.0% RH to 0% RH.
Lactose
Lactose powder (2.71 mg) was loaded into a metalized quartz pan and equilibrated at 25 °C. Following an initial drying period, the sample was ramped to 90% RH at a rate of 0.10% RH per minute.
Sodium Naproxen
Sodium naproxen powder (0.89 mg) was loaded into a metalized quartz pan and equilibrated at 25.0 °C. Humidity was set to 0% and, following an isothermal hold, humidity was stepped in increments of 5% to 95% RH. The initial desorption profile included stepping humidity in increments of 5% RH from 95% RH to 10 % RH. This adsorption-desorption profile was repeated for a second time. For isohume experiments, RH was held at 65% and temperature was increased gradually from 25 °C to 50 °C.
Results and Discussion
Assessing Hygroscopicity
Raw materials and APIs are unavoidably exposed to humidity during processing and storage, which can lead to profound changes in the drug product. Generally, the more hygroscopic a material is, the more likely its properties are to be influenced by moisture; therefore, it is critical to profile the hygroscopicity of active and inactive ingredients (3). Sorption-desorption experiments are an effective test to determine if a material is susceptible to the effects of moisture. Figure 2 shows the weight gain of ibuprofen when it was exposed to a gradual increase in humidity from 0% to 90% RH at 25 °C. Despite slight hysteresis, ibuprofen shows mass loss when humidity was decreased from 90% to 0% RH, indicating that the moisture sorption can be reversed.
Table 1 shows the classification of hygroscopicity as defined by the European Pharmacopeia (4). Due to its 5.5% weight gain from 0% to 80% RH, ibuprofen is classified as moderately hygroscopic. If different drug candidates with the same pharmacological effects are available, selecting the less hygroscopic substance will result in increased stability.
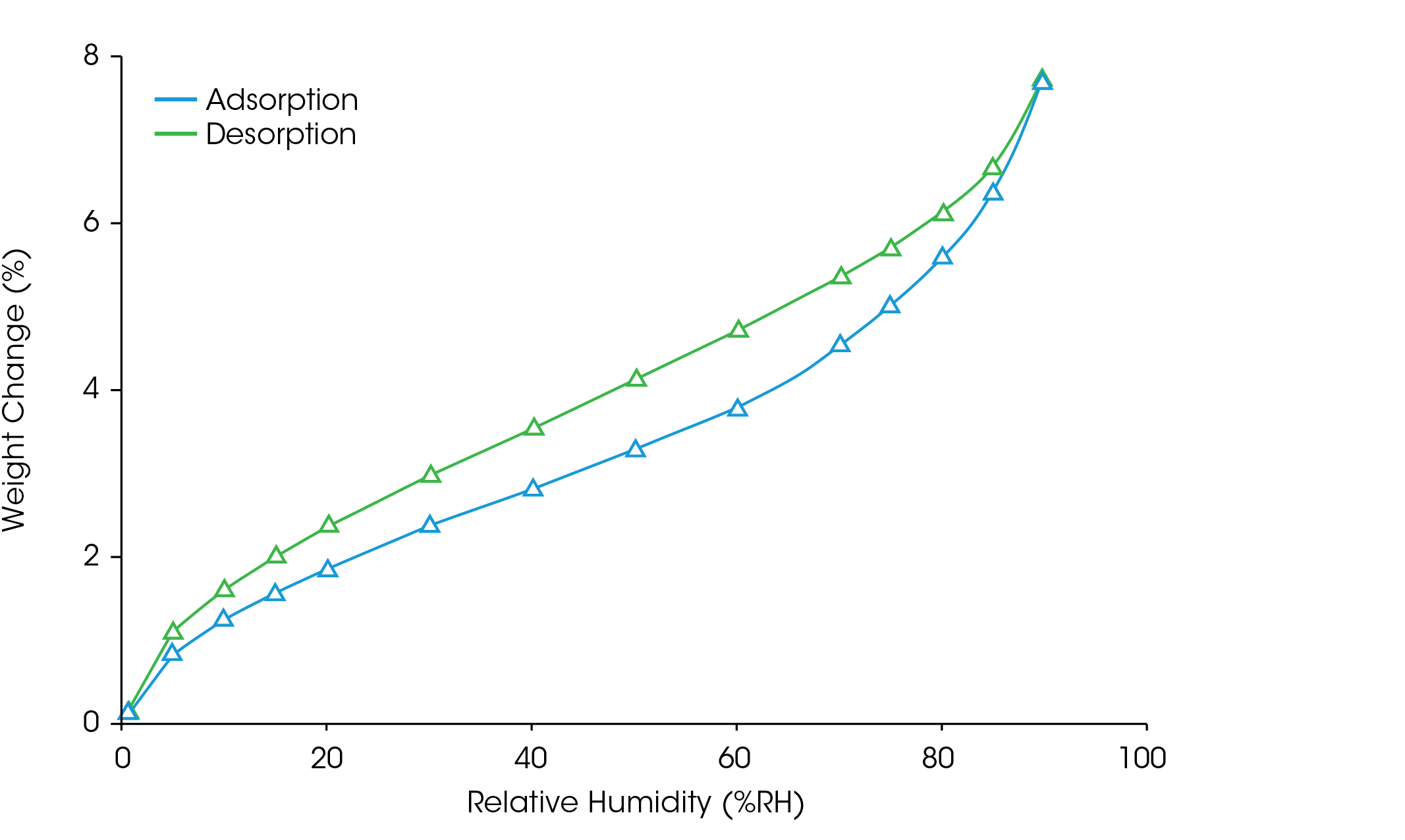
Table 1. Hygroscopicity class definitions as provided by the European Pharmacopeia
| Hygroscopicity Class | Percent weight gain at 25 °C, 80% RH |
|---|---|
| Non-Hygroscopic | 0 – 0.12 |
| Slightly Hygroscopic | 0.2 – 2.0 |
| Moderately Hygroscopic | 2.0 – 15.0 |
| Very Hygroscopic | > 15.0 |
Assessing Amorphous-Crystalline Phase Changes
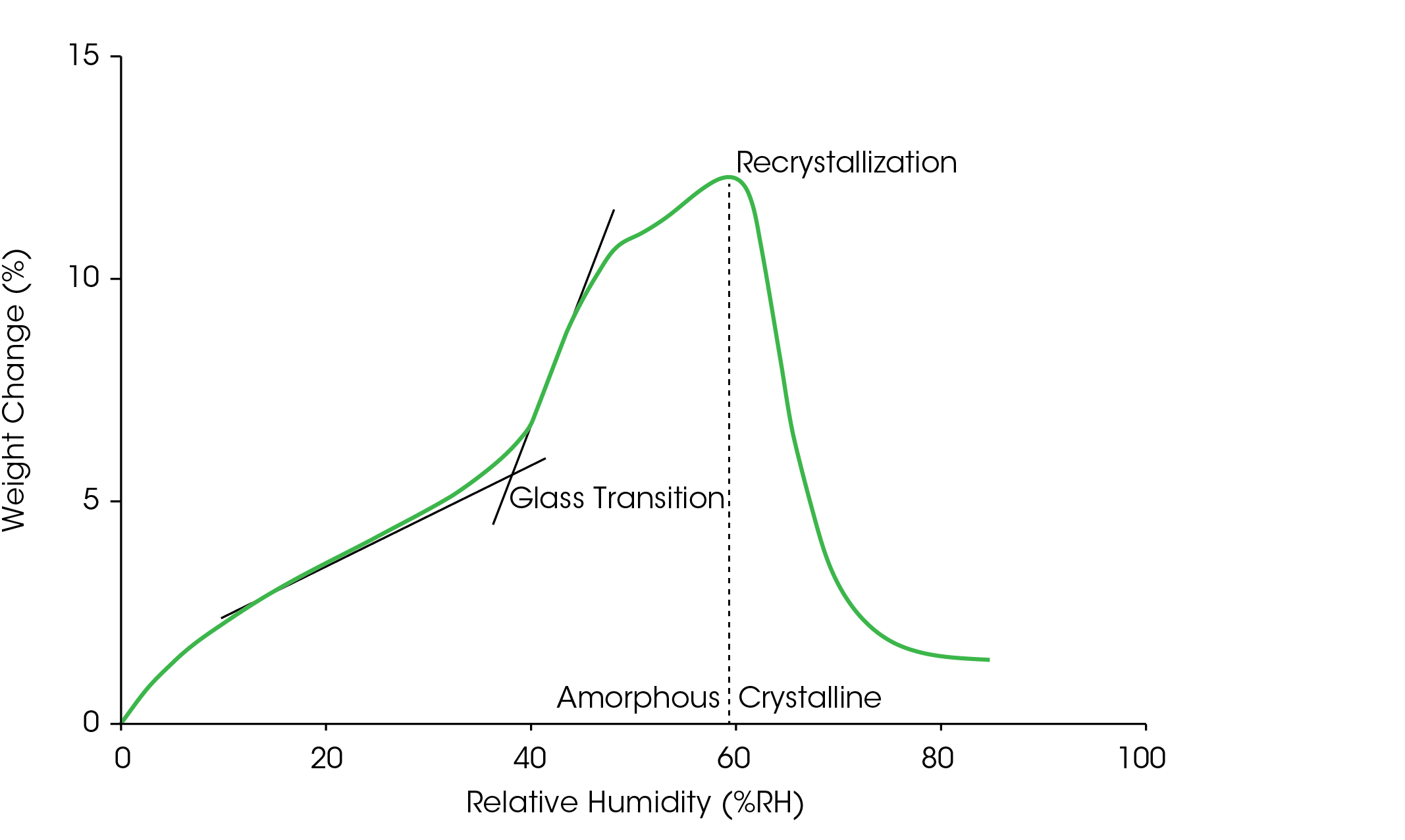
It is critical that the desired structure of the API or excipients be consistent throughout processing and storage; therefore, it must not be susceptible to a phase change over the humidity conditions it is anticipated to experience. Moisture adsorption can affect the Tg of an amorphous structure, which could ultimately cause a phase change in the material from amorphous to crystalline (5). Lactose is used commonly as a filler or diluent in tablets and capsules, and, as shown in Figure 3 , at 25 °C, amorphous lactose undergoes a glass transition when the RH approaches 40% that further increases its hygroscopicity. Increasing the RH to 60% drives the lactose to recrystallize that result in a corresponding decrease in hygroscopicity, as evidenced by the drop in weight between 60% and 80% RH. Crystallization will decrease the solubility of a material; therefore, the substance must be processed and stored at or below its recrystallization conditions. During formulation, it is especially important to characterize both API and excipient, because reduced solubility will affect the bioavailability of a drug.
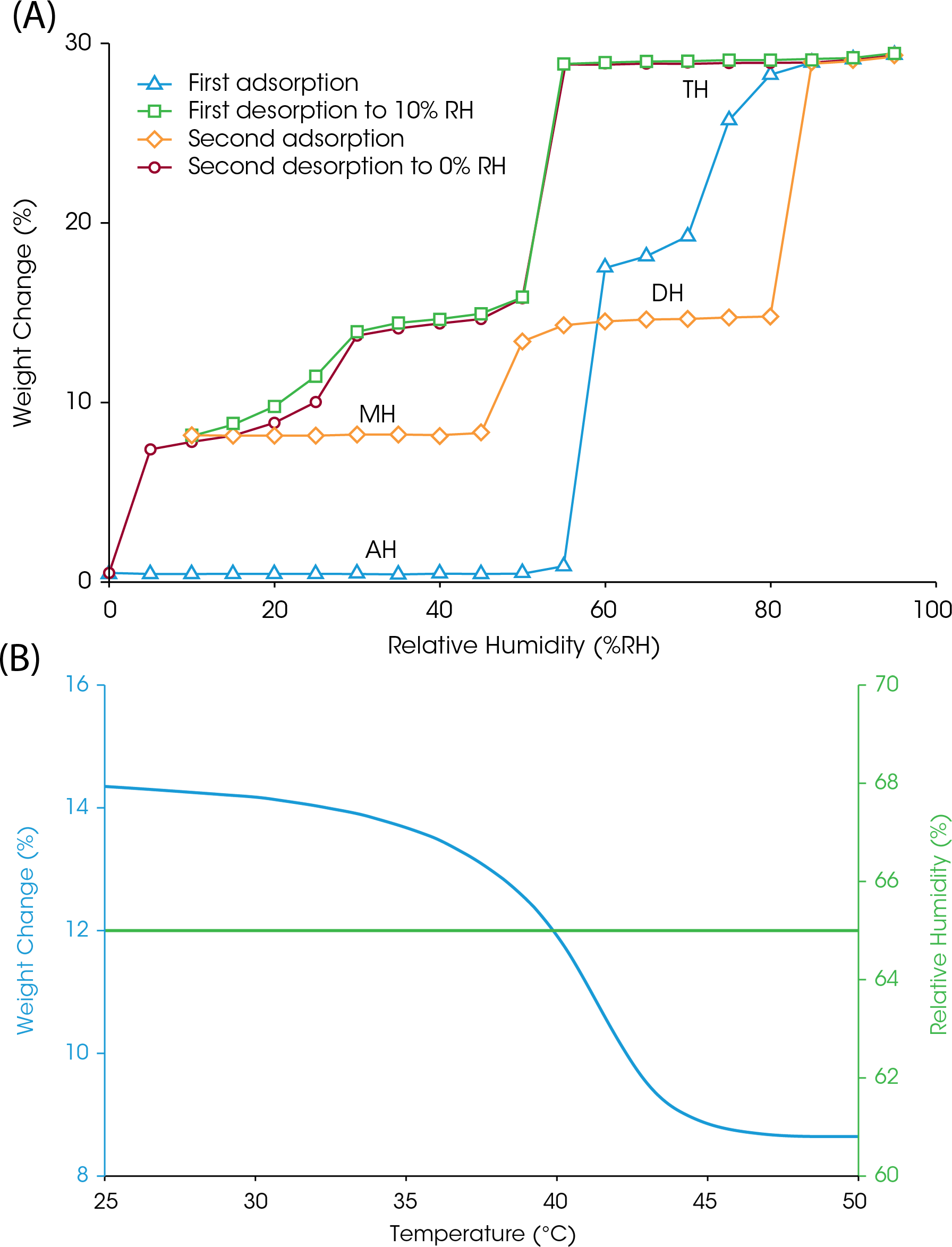
Exposure to humidity at any stage of the drug production process can cause spontaneous hydrate formation, which will affect the physical properties of the API (6). Properties affected by hydrate formation may include its solubility, dissolution ability, and bioavailability. Sodium naproxen presents a case study for how the Discovery SA can reveal and detect multiple hydrate formations for a single drug compound. Figure 4A shows the change in weight as sodium naproxen undergoes two adsorption-desorption cycles at 25 °C. Beginning at 0% RH, the anhydrous form (AH) resists water adsorption until the atmosphere approaches 60% RH and experiences a subsequent weight gain roughly at 80% RH. The desorption profile reveals three distinguishable weight loss events, suggesting that sodium naproxen forms a monohydrate (MH), dihydrate (DH), and trihydrate (TH) depending on the RH conditions, a pattern that repeats itself during the second adsorption-desorption cycle.
The Discovery SA can also be used to determine the effects of temperature change when humidity is held constant. Drug compounds can also experience hydration or dehydration events when humidity is held constant, and temperature is changed. When RH is maintained at 65% and temperature is increased, the sodium naproxen dihydrate will experience weight loss between 30 – 45 °C (Figure 4B), revealing the interrelatedness between temperature and humidity, and the role they play in hydrate formation. Each hydrate has its own unique physical properties that could affect the efficacy of a drug product, which highlights the importance of performing a thorough investigation of its adsorption and desorption tendencies.
Conclusions
The interaction between moisture and a drug product can have tremendous consequences on its structure and stability, which can impact its efficacy and shelf life. The Discovery SA offers precise environmental control that allows the effects of humidity and temperature to be quantified with the enhanced precision.
References
- Lee, S., Dekay, H.G., Banker, G.S. Effect of water vapor pressure on moisture sorption and the stability of aspirin and ascorbic acid in tablet matrices. J. Pharm. Sci., 1965, 54(8), 1153-1158.
- Leeson, L.J., Mattocks, A.M. Decomposition of aspirin in the solid state. Journal of the American Pharmaceutical Association. 1958, 47(5), 329-333.
- Newman, A.W., Reutzel-Edens, S.M., Zografi, G. Characterization of the “hygroscopic” properties of active pharmaceutical ingredients. J. Pharm. Sci., 2008, 97(3), 1047-1059.
- Anonymous. Characters Section in Monographs. European Pharmacopoeia, 6m Version 6.8, Section 5. 11th Ed. Strasbourg, France: European Directorate for the Quality of Medicines & Healthcare (EDQM); 2010.Sheokand, S., Modi, S.R., Bansal, A.K. Dynamic vapor sorption as a tool for characterization and quantification of amorphous content in predominantly crystalline materials. J. Pharm. Sc., 2014, 103, 3364-3376.
- Jurczak, E., Mazurek, A.H., Szeleszczuk, L., Maciej, Pisklak, D.M., Zielinska-Pisklak, M. Pharmaceutical hydrates analysis – overview of methods and recent advances. Pharmaceutics, 2020, 12(10), 959.
Acknowledgement
For more information or to request a product quote, please visit www.tainstruments.com to locate your local sales office information.
This paper was written by Samuel Redstone using data collected by Carlton G. Slough and Hang Lau.
TA Instruments, Discovery, TRIOS, and Tru-Mass are trademarks of Waters Technologies Corporation.
Click here to download the printable version of this application note.

