Keywords: TAM, IMC, API, Stability, Compatibility, Polymorphism, Amorphicity, Crystallinity
MC159
Introduction
This application note will review the microcalorimetric techniques that are most commonly used within pharmaceutical science: including stability and compatibility tests, determination of small amounts of amorphic content and characterizing polymorphism.
A microcalorimeter can quantify the amount and rate of heat release from chemical or physical processes associated with stability and shelf life of a drug or a formulation. Examples include processes caused by the interaction of one component with another or physical processes as crystallization or polymorphic transformations. The data can be both qualitative and quantitative and can be used to determine relevant characteristics of a sample.
Microcalorimetry has the advantage of being general, non-destructive and very sensitive. The high sensitivity of the technique makes it possible to obtain reliable stability data within hours or days and close to ambient storage conditions. It is general in the way it can detect both chemical and physical changes within the sample, both being critical for the performance of the pharmaceutical. The non-destructive nature of the technique gives the possibility to further analysis of the sample after completion of the calorimetric measurement.
TAM is a flexible, and sensitive microcalorimetry system with an exceptional long-term measuring stability. TAM is a modular system consisting of a precisely controlled thermostat that can be equipped with a wide range of calorimeters, sample handling system and accessories to be able to conduct the required tests described in this application note.
Stability
Isothermal microcalorimetry can detect both chemical and physical changes within a sample, it is being used to obtain reliable stability data within hours or days and under near ambient storage conditions.
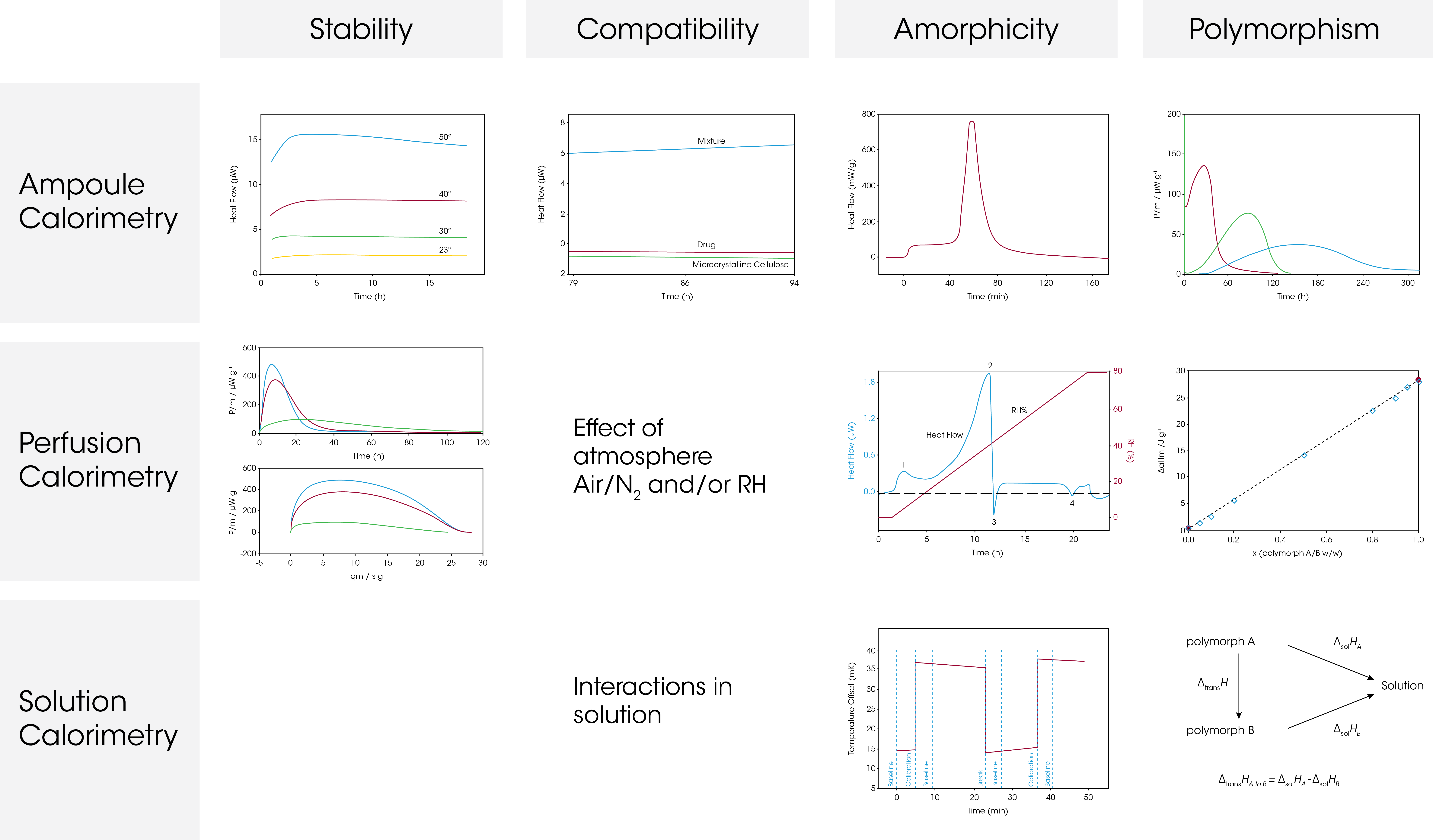
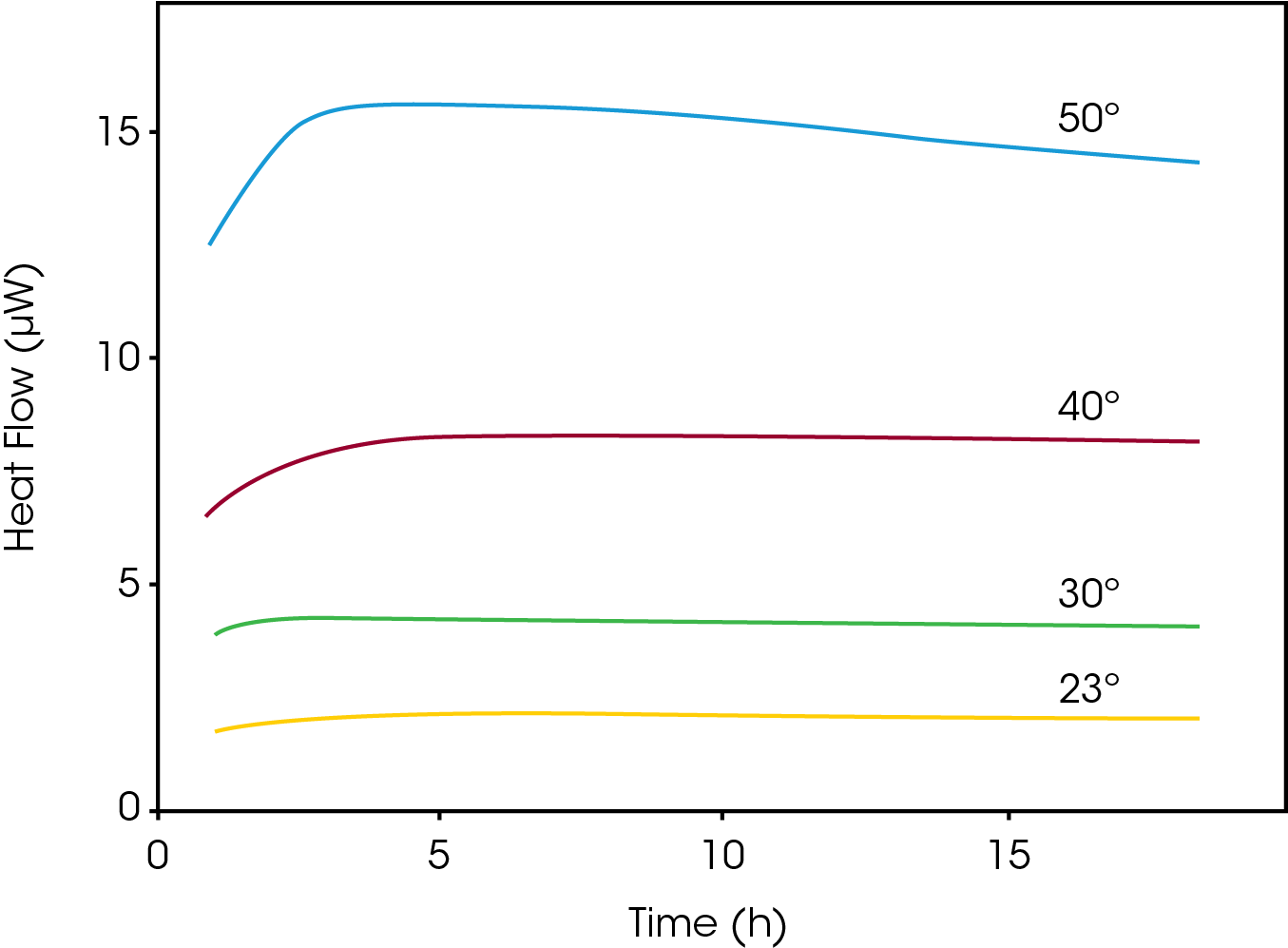
Chemical degradation is conventionally studied by HPLC, which has the drawback of being relatively insensitive to small changes in concentration. To accelerate any degradation reactions taking place in the sample and shorten the analysis time, elevated temperatures are often used. The data generated when using such accelerated timelines typically require extrapolation to predict the stability at the temperature selected for storage.
The figure below shows the oxidation of dl-α-tocopherol at different temperatures (50 °C, 40 °C, 30 °C and 23 °C). As the temperature increases, the heat flow curves get larger in magnitude, i.e., the rate of reaction increases. The heat flow data was fitted for first order kinetics and the rate constant was plotted in an Arrhenius relationship together with HPLC data on the oxidation product. The TAM data (circles) and HPLC data (triangles) fall into the same linear Arrhenius relationship, indicating it is the same reaction being detected, although TAM is capable of detecting the reaction at much lower temperatures.
Thus, microcalorimetry is particularly useful for quantifying very slow reaction rates.
Compatability
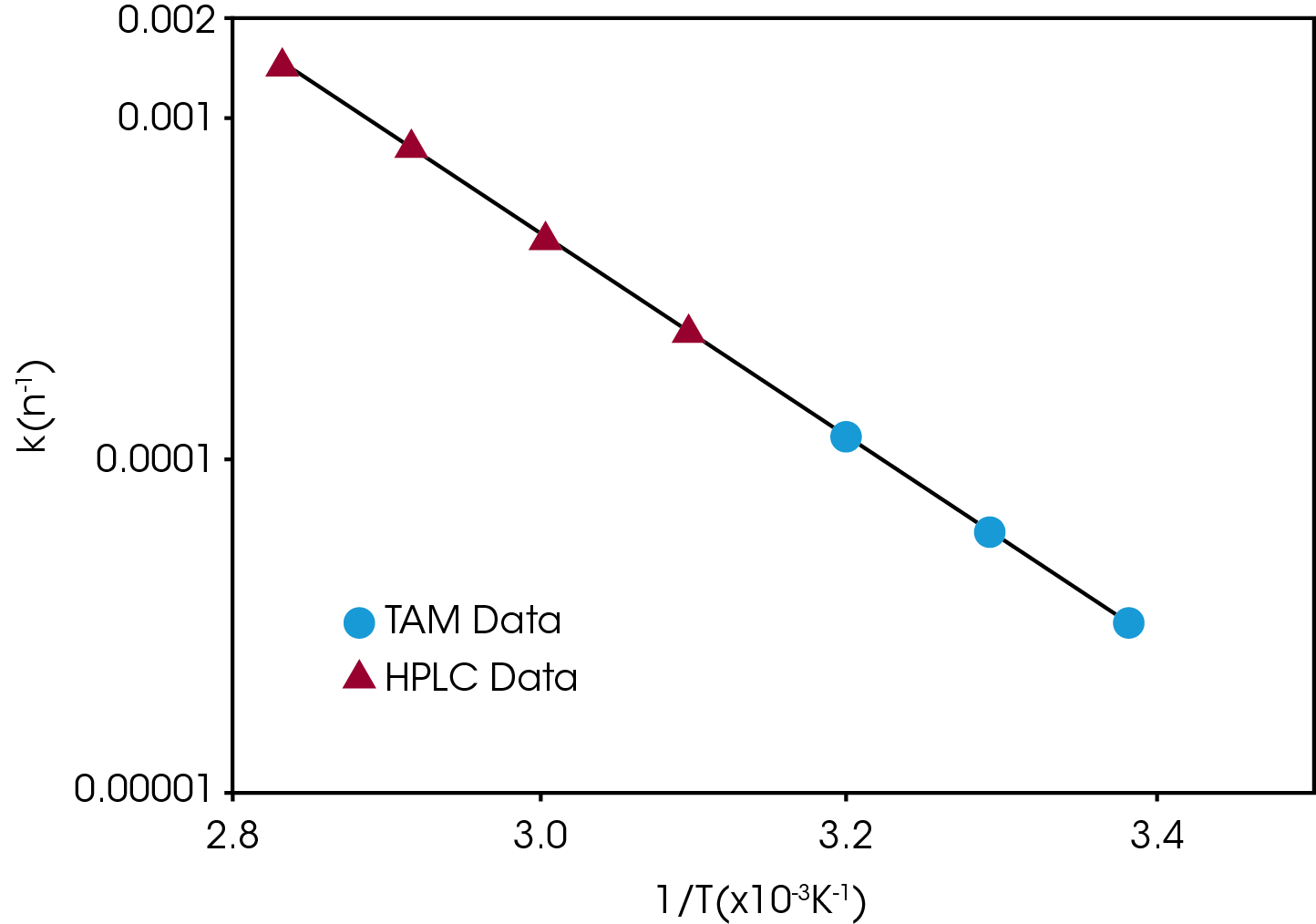
Compatibility testing is a form of stability testing that evaluates the possible interaction between the constituents of a multi-component sample, e.g., an API with an excipient. Microcalorimetry has proven to be particularly useful for compatibility testing; in some cases, data is obtained after only a few hours. The difference between the measured response and the expected or non-interactive response from combining the responses of the individual components indicates incompatibility.
Screening for stability and compatibility can be performed as stress tests with increased temperature (e.g., 40°C) and/or relative humidity (e.g., 50 or 75 %). The tests can be performed both qualitatively (Yes/No) and quantitatively (understanding the kinetics of the process) as it is possible to kinetically model the heat flow data. These tests can be performed on the API alone, in combinations with excipients – compatibility. It can also be performed on the formulated product as well as with packaging materials.
Amorphicity
The degree of crystallinity is a measure of crystal perfection. The crystallinity of all real crystals lies somewhere between that of a perfect crystal and a totally amorphous material corresponding to zero crystallinity. Crystals with a relatively small amount of imperfections are said to possess high crystallinity while crystals with high amounts of imperfections are said to have low crystallinity.
A pharmaceutical can be produced intentionally in an amorphous form for increased bioavailability. Stability needs to be demonstrated and controlled as discussed in the previous section. Small amounts of amorphous content in a supposedly crystalline material can be formed by processing e.g., by milling or compression and it is critical to detect and to be able to quantify.
Processed induced amorphous regions can have a great impact even if the percentage is small because these regions are typically located on the particle surface. The presence of imperfections (amorphicity) in a crystal affect properties as solubility, dissolution rate and surface energy and even small amounts need to be detected and sometimes quantified.
Imperfections in a crystal increase the energy (enthalpy) of the crystal. This enthalpy increase compared to a reference crystal of high crystallinity can be measured by calorimetry by two methodologies that are described in the USP 696 Characterization of crystalline solids by microcalorimetry and solution calorimetry:
- Heat of crystallization – induced by humidity (or solvent vapor pressure) by lowering the glass transition.
- Heat of solution by measuring the dissolution enthalpy.
Heat of crystallization with the micro-hygrostat method
A dry sample is inserted into a closed disposable glass ampoule together with a small tube partly filled with pure solvent or a saturated salt solution to give a certain relative humidity (i.e., vapor activity). The sample preparation takes place on the bench where after the ampoule assembly is thermally equilibrated and introduced into the measuring position of the calorimeter. Vapor from the liquid in the tube will evaporate and sorbs preferentially on the accessible amorphous regions of the particles. This will lower the glass transition temperature or increase the plasticity of the amorphous regions and a recrystallisation of the vapor accessible amorphous regions can be initiated provided that the vapor activity and the calorimetric temperature is high enough.
A typical recrystallisation result consists of an initial sorption period where after a sharp peak due to the crystallization process is obtained. The integrated value is directly related to the amount of amorphous material that has crystallized. For quantification the specific heat of crystallization must be known from a separate experiment with a known amount of amorphous material, e.g., a sample representing 100% amorphous material.
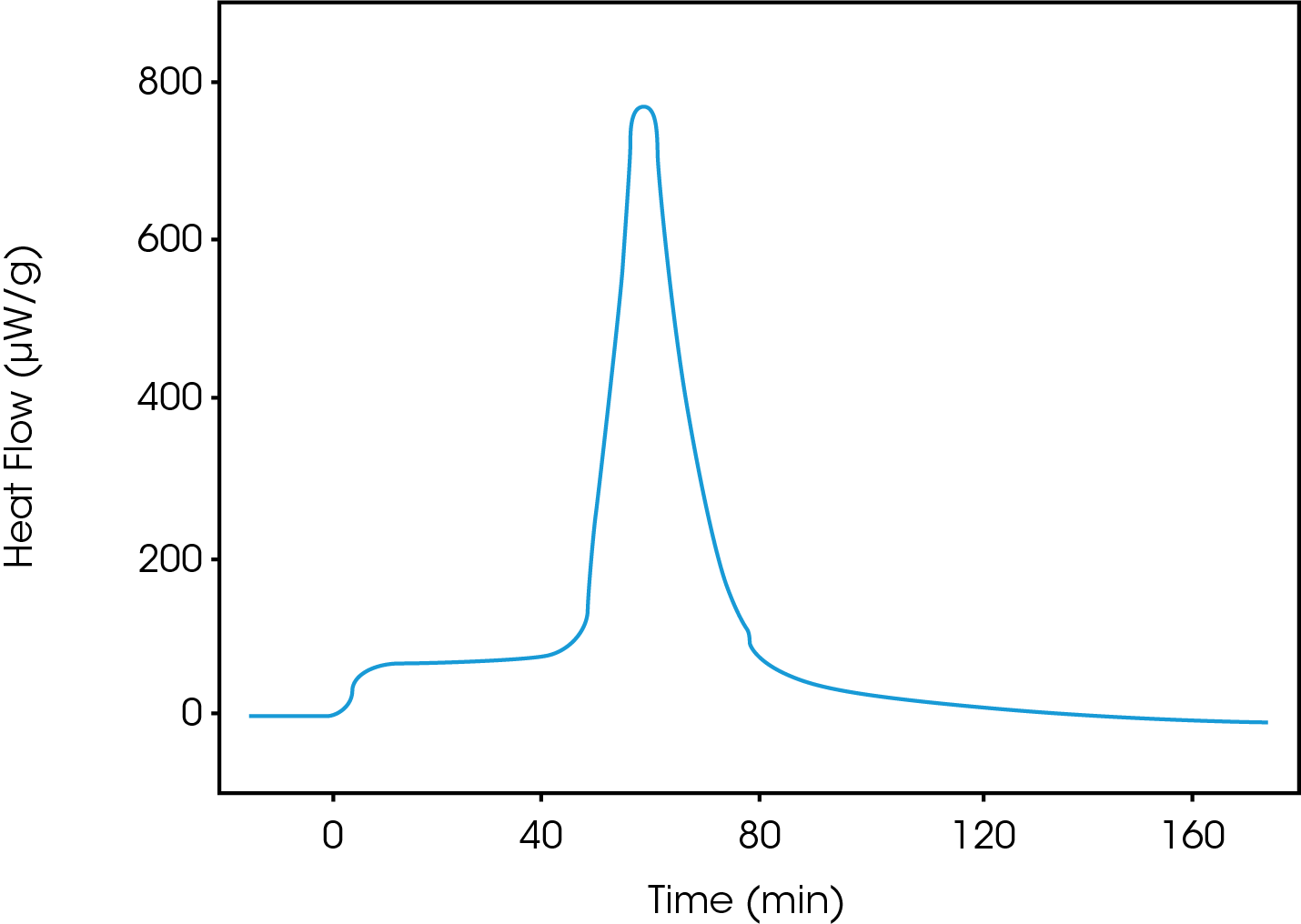

Heat of crystallization with the controlled relative humidity perfusion (cRHp) method
This technique can be very sensitive and fractions of a percentage of amorphous phases in crystalline samples can be detected. The sample is contained in a flow through ampoule where the vapor activity (e.g., RH) can be controlled and changed. Sample is equilibrated at a temperature and RH that do not induce crystallization. The RH is subsequently changed up and down in three equal and well-defined steps: The first step will involve absorption and crystallization, the second desorption and the third only adsorption. The difference between the integrated heat of the first and third peak is attributed to crystallization.
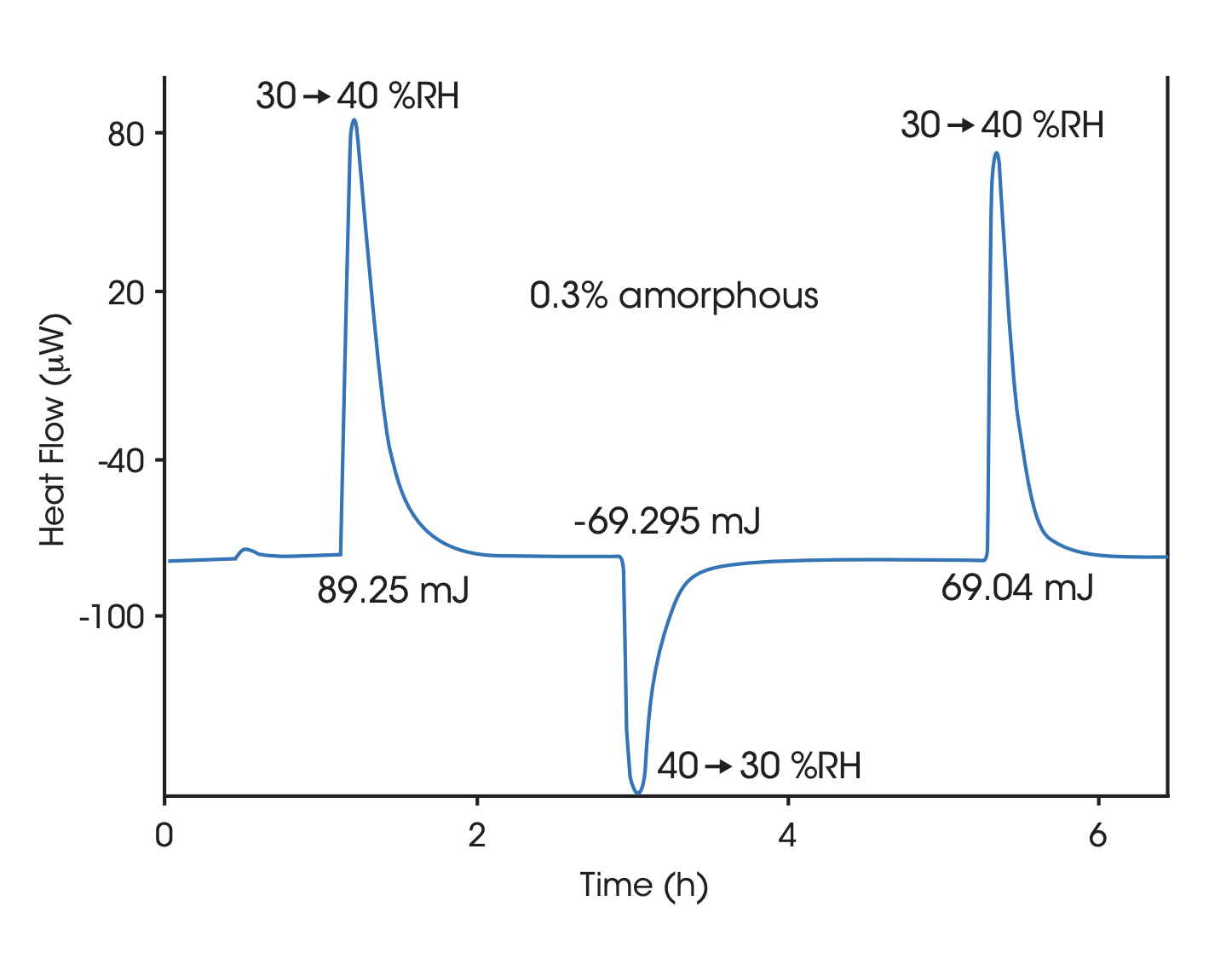
Heat of dissolution
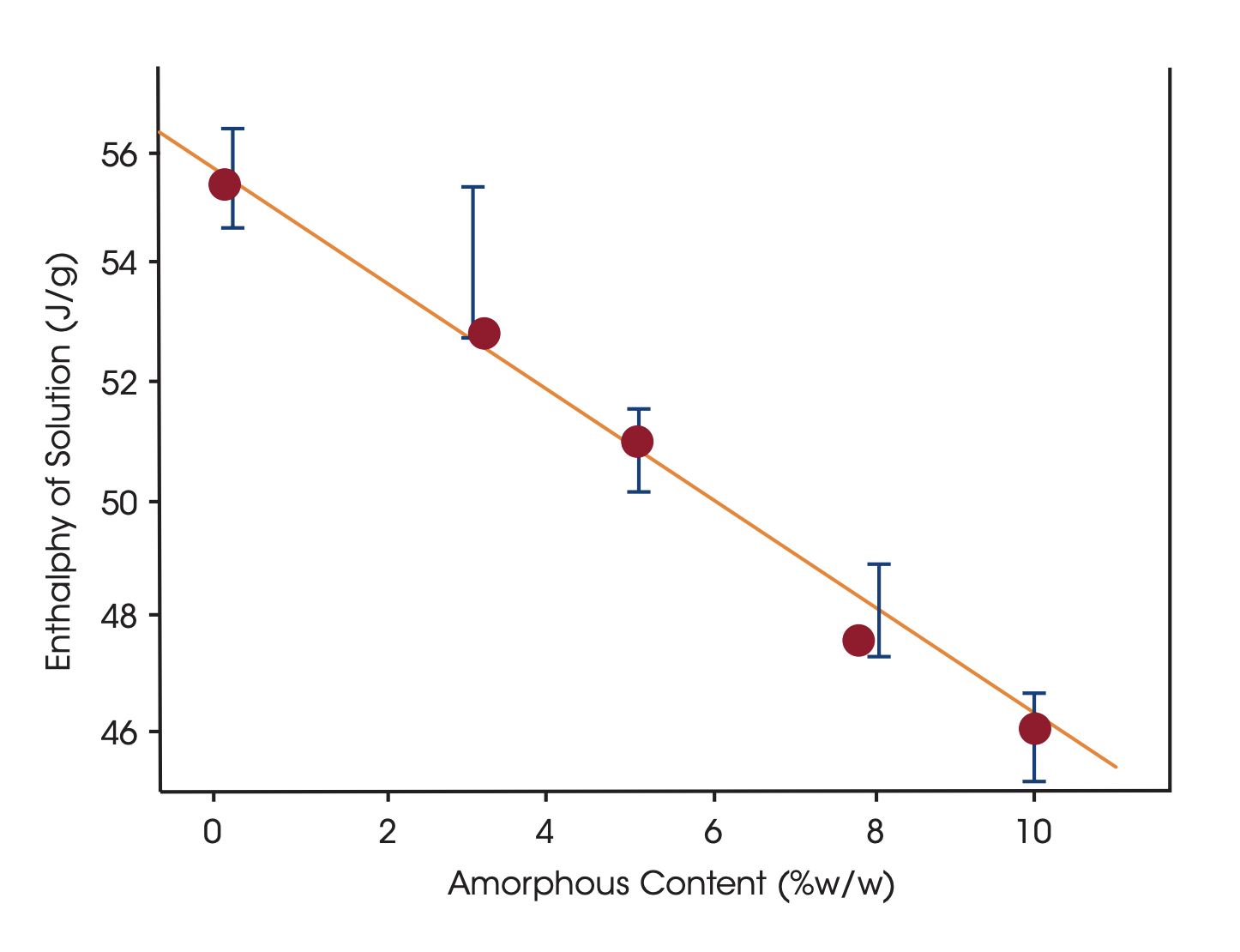
By dissolving the crystals in a suitable solvent in a Solution Calorimeter the crystal structure breaks up and the heat of dissolution is measured. The heat of dissolution is strongly related to the enthalpic content of the crystals. A higher enthalpic content relative to that of a perfect crystal correspond to a lower degree of crystallinity. To attain a quantitative measure of crystallinity the heat of solution of a reference material corresponding to a highly crystalline reference material and a material corresponding to 100% amorphicity must be determined in separate measurements.
| Technique | Sensitivity % amorphous content | Time for analysis | Sample amount needed | Throughput | Method setup and evaluation | Other |
|---|---|---|---|---|---|---|
| TAM – Microhygrost | Around 1% | 1-5 hours | 10-500 mg | 1-48 simultaneous measurements/maximum samples per day: 96 |
|
|
| TAM – cRHp method | Down to 0.1% | 5-20 hours | 10-50 mg | 1-4 simultaneous measurements/maximum samples per day: 2 (8) |
|
|
| TAM – SolCal method | Down to 1% depending on substance | 2 hours | 50-500 mg | 1-4 simultaneous measurements/maximum samples per day: 3 (12) |
|
|
Isothermal microcalorimetry has several benefits compared to other methods to assess amorphicity and these include sensitivity, versatility and sample throughput. A summary of the IMC methods can be seen in the table above:
Polymorphism
Polymorphism refers to the ability of a substance to crystallize into different crystal forms. Since the physical and chemical properties of a substance are closely related to its crystal structure, polymorphism is of great importance to activities related to solid-state materials.
The study of polymorphism and pseudo-polymorphism is a critical part of the drug development process because pharmaceutical properties can be impacted depending on which forms exist in the final product. For example, since polymorphs are in different energy states (including metastable forms), solubility can be affected, which in turn can impact bioavailability. Interconversion from a more soluble to a less soluble form may occur during manufacture of the pure drug, during formulation processes, and after long-term storage, thereby changing the pharmaceutically active properties of the final product.
With TAM it is possible to:
- Determine relative stability of polymorphic pairs
- Get transition temperatures (if solubility data exists)
- Answer the question is my powder in a meta-stable state?
- Process monitor: which factors can kinetically stabilize /destabilize the system
The thermodynamics and kinetics of transfer of one polymorphic form to another of lower energy is very important to quantify. For instance, the relative stability of two forms can be assessed by solution calorimetry. A “yes or no” answer as to whether a given form of a substance that might be meta-stable can be transformed into another form of lower energy.
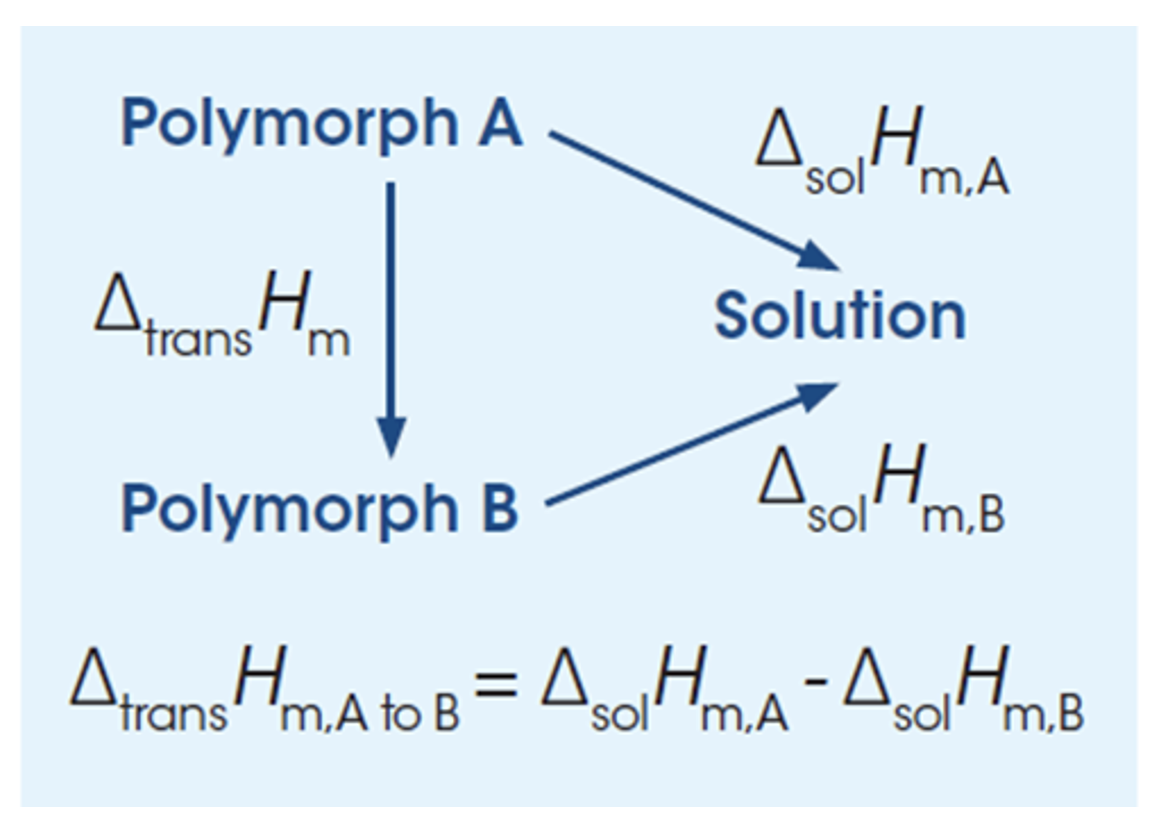
Calculations of the enthalpy of transition between two polymorphs from the heats of solution. By measuring the heat of solution of two different polymorphic forms, it is possible to calculate the transformation enthalpy (ΔtransH) for the transition of form A to form B by taking the difference in the heat of solution between the two forms (ΔsolHA and ΔsolHB). The direction of the transformation (A to B or B to A) is an indication of the relative stability of the pair and is more likely to occur if the transformation enthalpy is negative (exothermic). In addition, if the solubility of the two forms is known the thermodynamic transition temperature between the two forms can be obtained for enantiotropic (reversible) polymorphic pairs.
Ampoule calorimetry can be used to study the kinetics of transition of one form into another in terms of the heat flow as a function of time. Transformation of a meta-stable form into the thermodynamically more stable form can occur either directly in the solid state or via a solvent phase in slurry (solvent mediated polymorphic transition).
Direct transformations in the solid state are in many cases very slow or too insignificant to observe in a calorimetric experiment. The slurry method can provide a “yes or no” answer as to whether the studied polymorphic form is in a meta-stable state.
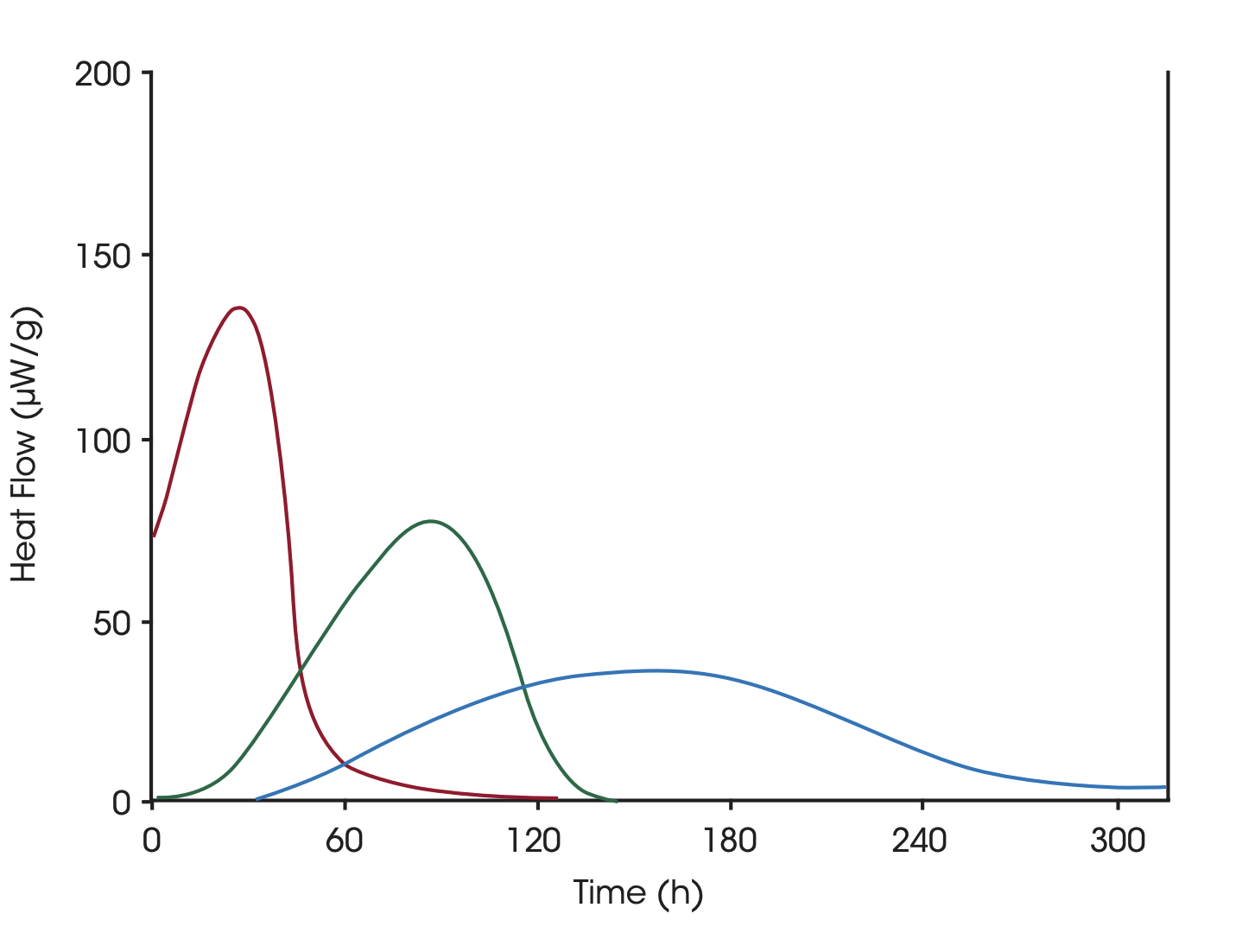
The solvent mediated process increases the rate of the process and works by adding a small amount of solvent to the sample. The least stable polymorph will be dissolved into a saturated solution and will crystallize into the more stable form. This reaction will continue until the sample has been completely transformed. The plot shows the polymorphic transformation for different lots of a drug that have been generated with different processes, blue: lot A, green: lot B, red: micronized lot A. The calculated enthalpies are the same, but the reaction rates are different due to differences in the stability of the samples.
Another application is to use the slurry method as a model system for studying the stability of a thermodynamically non-stable form under a varying set of conditions such as temperature and the chemical environment (e.g. presence of impurities or excipients).
Summary
Isothermal microcalorimetry and TAM is an acknowledged tool for some specific applications in the pharmaceutical industry. These applications are particularly relevant to pre-formulation studies, formulation development and analytical development. In these environments, techniques based on TAM are used to assess chemical stability of drugs, stability of polymorphs, drug-excipient compatibility and degree of crystallinity.
TAM offers non-destructive real-time and continuous measurements of chemical and physical processes at high sensitivity and with possibility of high sample throughput.
Acknowledgement
This application note is a summary of several TA Instruments applications notes and was written by Malin Suurkuusk at TA Instruments.
TA Instruments has been long recognized as an innovator and leader in modulated thermal analysis.
Click here to download the printable version of this application note.

