Keywords: DSC, TGA, Sorption Analysis, Thermal Analysis, Pharmaceuticals, Melting, Crystallization, Thermal Stability, Compatibility, Glass Transition, Hygroscopy, Moisture, Wetting, Drying
TA484
Abstract
Sales in the German pharmaceutical market amounted to around 56.5 billion euros in 2022. This means that the volume of the pharmaceutical market in Germany has more than doubled in the last fifteen years, making Germany the largest pharmaceutical market in Europe [1]. The pharmaceutical industry is therefore a significant economic factor and important employer in Germany. Drug manufacturers must follow guidelines that control facilities and methods used in the processing, manufacturing, and packaging of a drug product. Careful monitoring of these processes is required and utilizes a variety of examination methods and controls.
Introduction
To ensure the consistent quality of final products, the raw materials must be checked and analyzed as part of the incoming goods inspection. On the other hand, there are a variety of analytical tasks involved in the research and development of new active ingredients and products.
When it comes to new active ingredients, a distinction is made between:
- Chemically defined substances
- Materials based on natural products
- Biopharmaceuticals
- Analogous active ingredients (modification of known active ingredients)
In pharmaceutical development, you are always looking for:
- New dosage forms and combination of active ingredients
- Possibilities for expanding the area of application
- Improvements in the production process, such as reducing energy consumption.
For this purpose, characterization methods that are as quick and simple as possible are needed. Thermal analysis techniques include:
This application note provides an overview of the various thermal analysis techniques and demonstrates the value these methods can provide for pharmaceutical samples.
Discussion
Thermogravimetric Analysis (TGA)
TGA determines the change in mass of a sample while it is heated or kept isothermal in a controlled atmosphere. A TA Instruments™ Discovery™ TGA system consists of the weighing system, the furnace, and a device for setting the controlled atmosphere. Temperature and mass are recorded. When a material is heated, most samples show a loss of mass. Less commonly, one sees an increase in mass due to oxidation under the influence of oxygen.
A TGA analysis can provide the following information:
- Loss of volatile fractions – water or solvent residues
- Thermal stability of materials
- Oxidative stability of materials
- Quantitative composition of multi-component systems
- Information on decomposition kinetics
- Expected service life

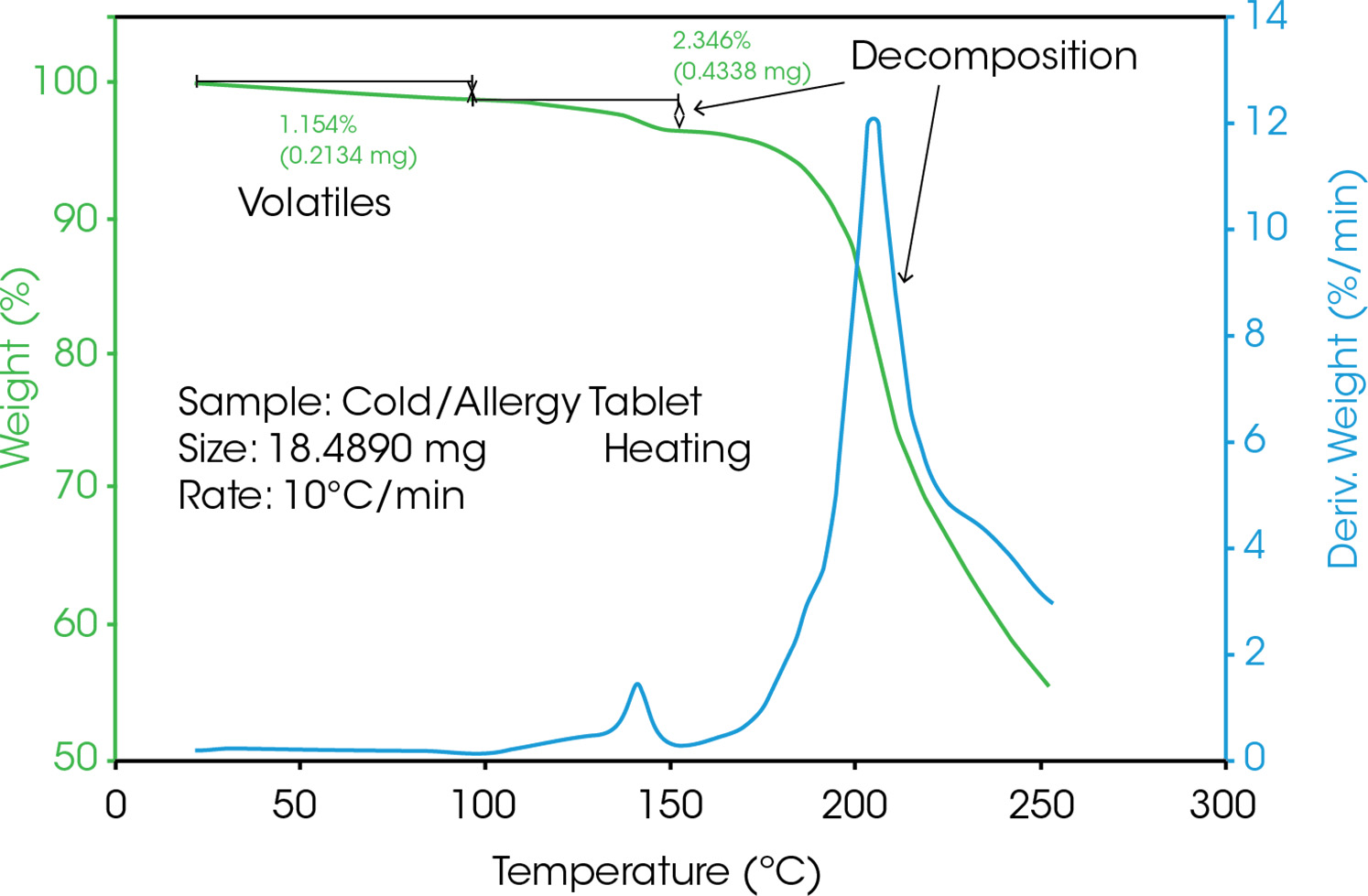
Figure 3 shows the TGA curve of an allergy tablet. In a TGA measurement, the mass (in %) above the temperature (in °C) is typically plotted, the dissipation signal (blue) provides additional information about the reaction rate between effect. The temperature at which highest reaction rate is highest can be found at the maximum. The minumum of the derivative (lowest reaction rate between two effects) are used to determine the evaluation limits of two consecutive stages. The curve first shows the loss of moisture, followed by multi-stage decomposition.
It is possible to couple a TGA system with a gas analysis, such as MS/FTIR/GC-MS. The escaping gases are transferred to the gas analysis with the purge gas stream, so that volatile components and decomposition products can be qualitatively identified.
Since the systems can be started simultaneously via a trigger signal, the data can be plotted together on a time basis.
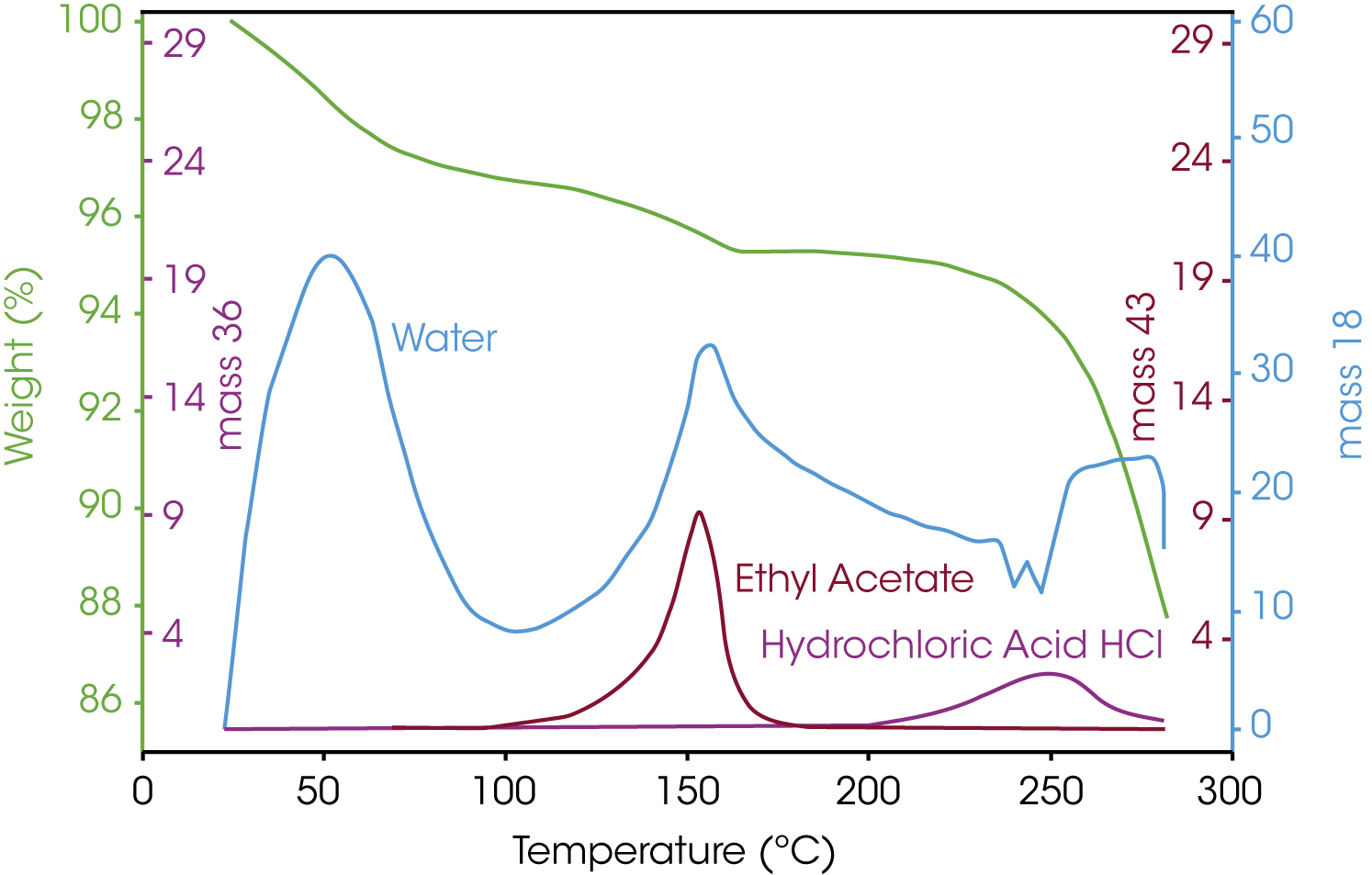
Figure 4 shows the superposition of the TGA curve with the masses 18, 36, and 43 detected in the mass spectrometer. In this way, the different degradation stages can be assigned to the solvents.
Sorption Analysis
The moisture content of solid dosage forms is one of the main factors influencing stability. A Dynamic Vapor Sorption Analyzer (DVSA) is used to determine the change in mass under water (Steam/Relative Humidity) and can be characterized by:
- Adsorption (mass increase)
- Desorption (mass decrease)
The determination of the mass change takes place as a function of the relative humidity (RH) and the temperature (T). The TA Instruments Discovery SA instrument uses a double humidity chamber instead of the TGA furnace and can be used in the range of 5–85 °C. By mixing a dry and a moist gas stream, relative humidity can be set from 0–98%.
This setup allows different interactions with water to be analyzed:
- Wetting
- Drying
- Hygroscopy
- Hydrothermal stability
- Hydrate formations
- Moisture-induced structural changes

Water vapor sorption depends on the structure of the material. Chemically identical material usually absorbs more water when it is in an amorphous state, compared to the crystalline structure.
Water sorption can significantly lower the glass transition temperature and initiate undesirable (re-)crystallization. The physical properties of the amorphous and crystalline phases are very different and can affect different product properties:
- Mechanical (pouring behavior)
- Physical (solubility)
- Chemical (stability)
- Pharmacological (bioavailability)
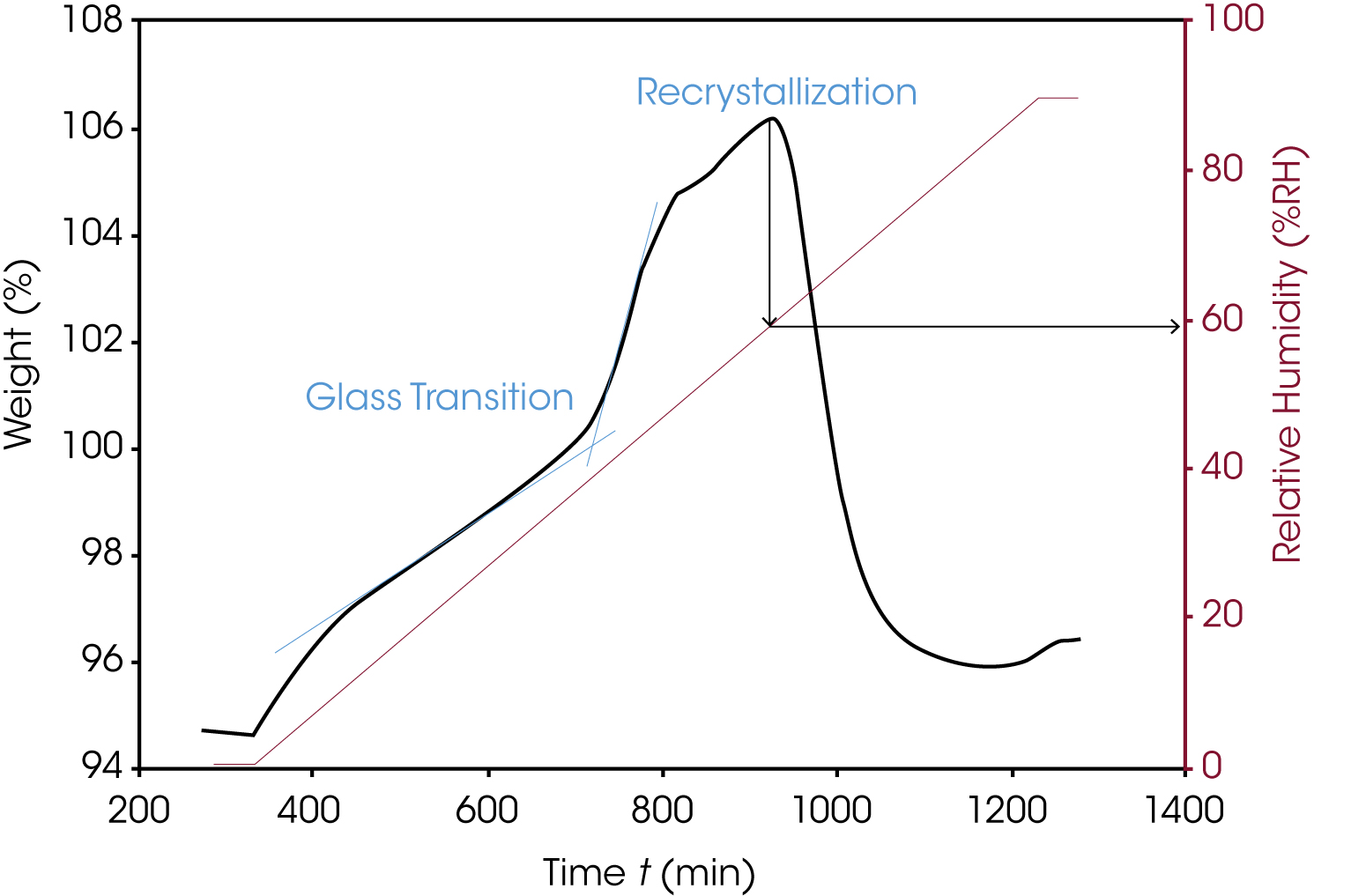
Sorption analysis is used, for example, to determine the relative humidity and temperature conditions at which a material crystallizes. The amorphous lactose sample shown in Figure 6 absorbs more water at 25 °C above the glass transition, which leads to recrystallization. This information is important to determine storage and transport conditions.
Differential Scanning Calorimetry (DSC)
DSC determines the heat flux that is released or absorbed as a function of time, temperature, and atmosphere. It is always measured against a reference sample, usually an empty pan. Open, closed, or hermetically sealed pans are used as sample vessels, and aluminum pans are most used.
The measurement can be carried out in heating, cooling, or isotherm, so the DSC system is typically combined with a cooler (compressor cooler or liquid nitrogen).
The heat flow shows:
- Phases or structural changes
- Chemical reactions and physical interactions
DSC can be used to determine material parameters, such as:
- Melting temperature
- Melting enthalpy
- Glass transition temperature
- Influence of cooling rate and storage on the formation of the amorphous and crystalline phases
- Purity determination
- Compatibility
- Polymorphism
- Denaturation

Compatibility
Incompatibility between any of the components can affect durability or effectiveness of the product. A determination of the individual components in comparison to a mixture of the components can provide information about this.
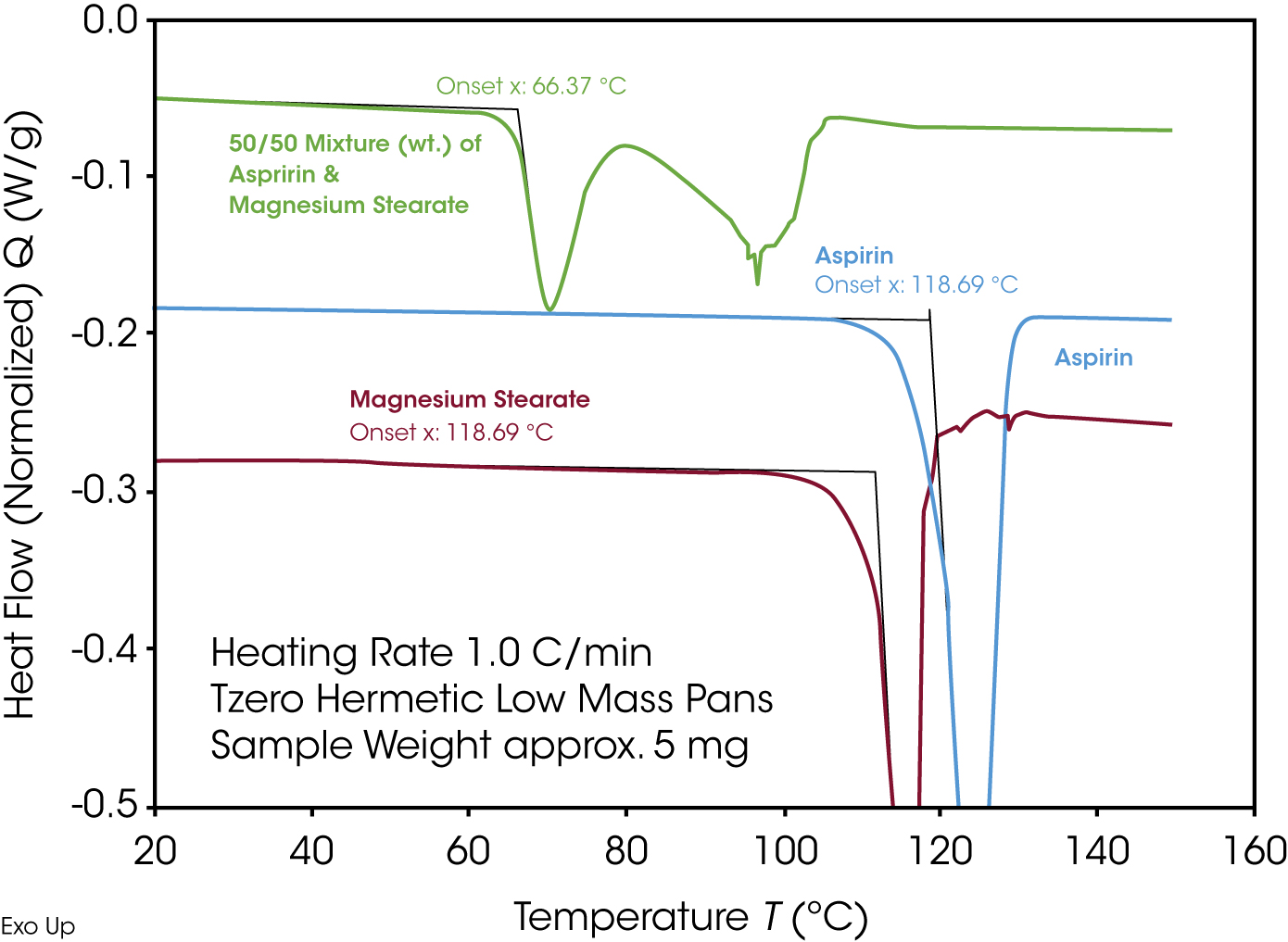
Magnesium stearate, often used as a lubricant in pharmaceutical tablet production, is shown as an example of compatibility testing using TA Instruments Discovery DSC in Figures. 8 and 9. Figure 8 shows the comparison of the 50/50 mixture of aspirin and magnesium stearate with the pure materials. The individual components each show an endothermic melting peak. If compatible, the mixture would show two melting peaks of proportional size in the same temperature window. However, the mixture already shows two endothermic effects at significantly lower temperatures – this is a clear indication of incompatibility.
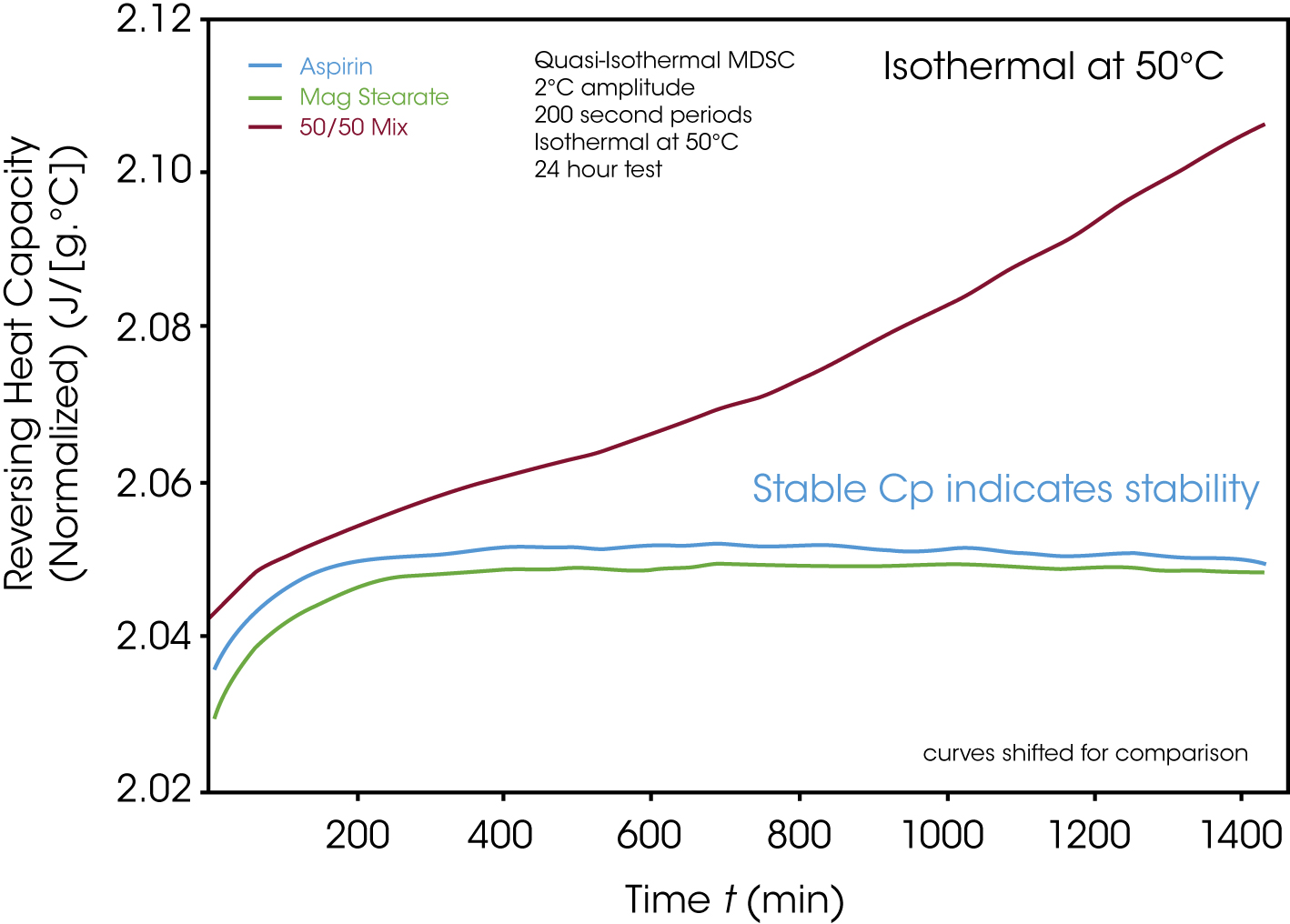
In order to check whether materials are compatible even at room temperature or below the melt, the heat capacity of the materials can be determined as a single substance and, in comparison, as a mixture instead of the heat flow. Modulated DSC™ (MDSC™) experiments offer great advantages here. This technique, in which a sinusoidal temperature oscillation is abandoned, allows the isothermal determination of the heat capacity (Cp). Figure 9 shows the stable heat capacity curves of the individual substances taken over time at 50 °C. The mixture is different: the heat capacity
Glass Transition
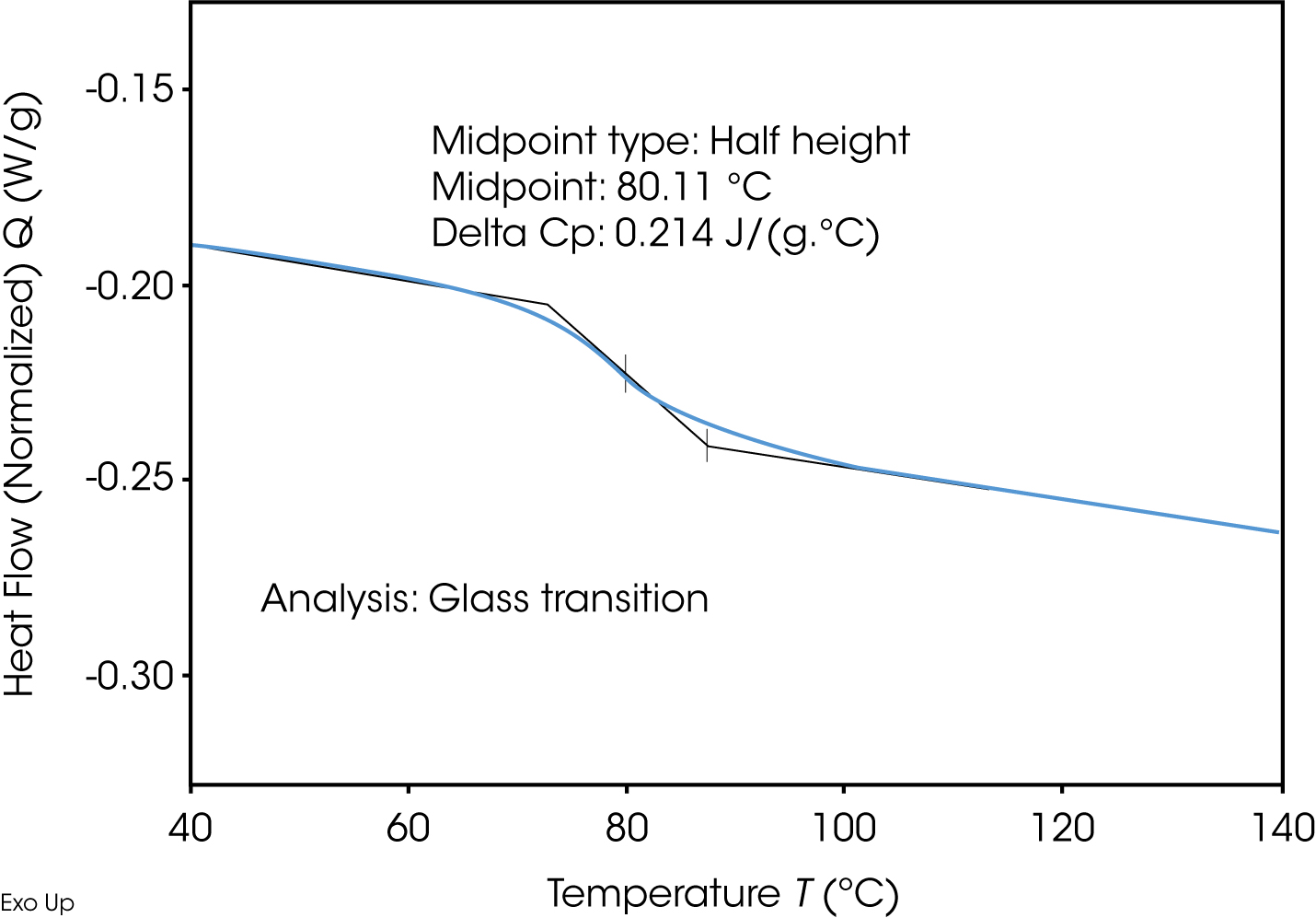
Another important aspect of analyzing materials for pharmaceuticals is the determination of the glass transition by means of DSC: The proportion of the amorphous phase of a pharmaceutical active ingredient or product has an influence on tabletability, solubility and thus on the release rate and bioavailability. In the case of amorphous or semi-amorphous substances, there is a risk of recrystallization due to their thermodynamic instability.
Due to the influence of humidity, the glass transition temperature can drop below the ambient temperature, potentially inducing crystallization. Crystallization can lead to the formation of polymorphic modifications or even hydrates, and agglomeration can form.
Absorption of moisture can change the physical properties of the dosage form. The glass transition occurs in the DSC signal as a step-like increase the temperature position and step height are of interest, as the properties changes can make a statement about the amorphous part.
Summary
Thermal analysis offers a wide range of possibilities with the methods TGA, SA, and DSC to determine material properties of active pharmaceutical ingredients, carrier materials, and end products. The methods require only a small amount of material, which is particularly advantageous in the field of research when only a small mass is available. Compared to the other methods, the DSC and TGA methods are relatively fast measurement techniques. Important sample information can be obtained in a short measurement time.
Acknowledgements
For more information or offers on our products, visit our website www.tainstruments.com or contact your local office.
This article was written by Monika Schennen.
TA Instruments, Modulated DSC, MDSC, and Discovery are trademarks of Waters Corporation.
References
- https://de.statista.com/statistik/daten/studie/158096/umfrage/pharma-gesamtmarkt-umsatzentwicklung-seit-2006
Click here to download the printable version of this application note.

