Keywords: Breast Implant Test, Mammary Implants, Breast Augmentation, ISO 14607, ASTM F703, ASTM F2051
EF001
Introduction
Implantable medical devices such as breast implants must be thoroughly evaluated for performance and reliability in many aspects. TA Instruments’ 3300 and 3510 load frames can be used to characterize the mechanical strength and durability of such devices. These insights are crucial for both the design optimization and final validation that is required for regulation.
Reduce the Risk of Breast Implant Failure
According to the International Society of Aesthetic Plastic Surgeons (ISAPS), almost 1.9 million breast augmentations were performed globally in 2018, accounting for approximately 18% of all cosmetic surgical procedures that year.1
Breast implants, typically consisting of an elastomeric outer shell and filled with either silicone or saline, must meet various standards and guidelines to ensure patient safety over time. Mechanical test instruments from TA Instruments can be used to characterize material properties of the implant shell or mechanical performance of the entire implant.
Testing Versatility
Test instrument configurations can be used for static and dynamic characterization of implants. Tests include rupture testing, elongation, compressive fatigue, and dynamic mechanical analysis (DMA).
Superior Waveform Fidelity
Frictionless actuator technologies provide the control and waveform fidelity needed when dynamically testing viscoelastic materials which can often be loading-rate sensitive.

Reliable for Billions of Cycles
All-electric test instruments can easily and efficiently run millions of fatigue-life tests without maintenance and are guaranteed by the testing industry’s only 10-year actuator warranty.
Achieve the Results Required for Device Approval
TA Instruments mechanical test solutions can perform many of the test methods recommended for regulatory approval of breast implants, such as:
- Quasi-static failure tests on dog-bone samples from shell materials including breaking strength, modulus, tensile set and seam integrity as outlined in ASTM F703, ASTM F2051 and ISO 14607
- Fatigue resistance of the implant can be evaluated by performing a complete device fatigue test-to-failure as defined in ISO 14607 Annex C or similar force-control methodologies
- Testing per US FDA Guidance for IDE/PMA applications for Saline, Silicone Gel, and Alternative Breast Implants2 which provides recommendations for compressive fatigue tests to generate applied force versus number of cycles to failure (AF/N) curves.
Example Test Data
One example is a complete device compression test-to-failure as described in ISO 14607. An illustration of the results from this monotonic, single load to failure test are shown in the Figure 1 where compressive rupture of the implant can be seen in the abrupt truncation of the curve. The maximum force before rupture was approximately 6,000 N of compression.
Cyclic compression testing is a fatigue test method that is aimed at characterizing the durability of implants. Example data is shown illustrated in Figure 2, cyclic loading in force control from -22.5 N to -225 N at a frequency of 3.33 Hz. This force control is executed until the device fails due to fatigue rupture, or until a satisfactory number of cycles (run-out) is obtained.

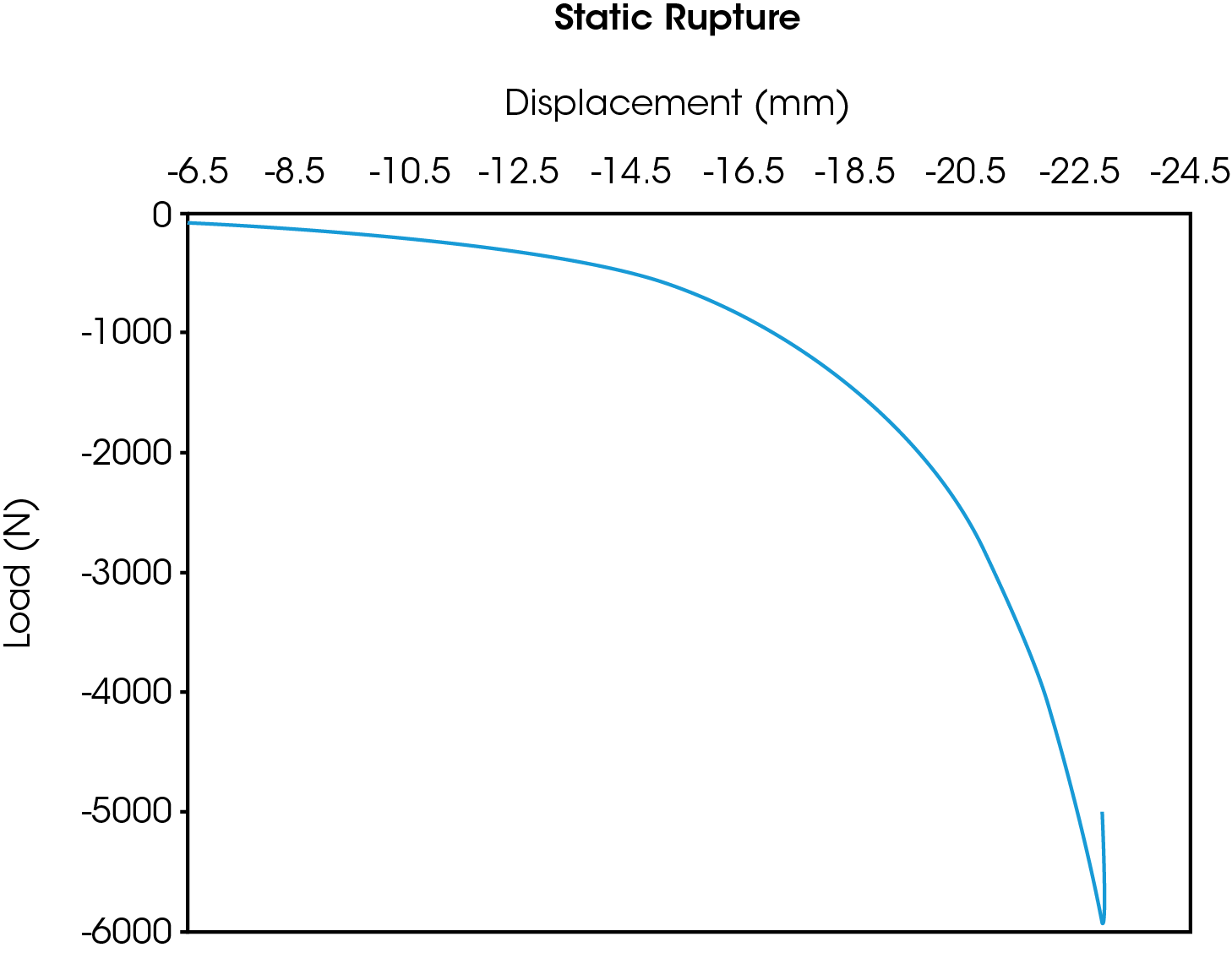
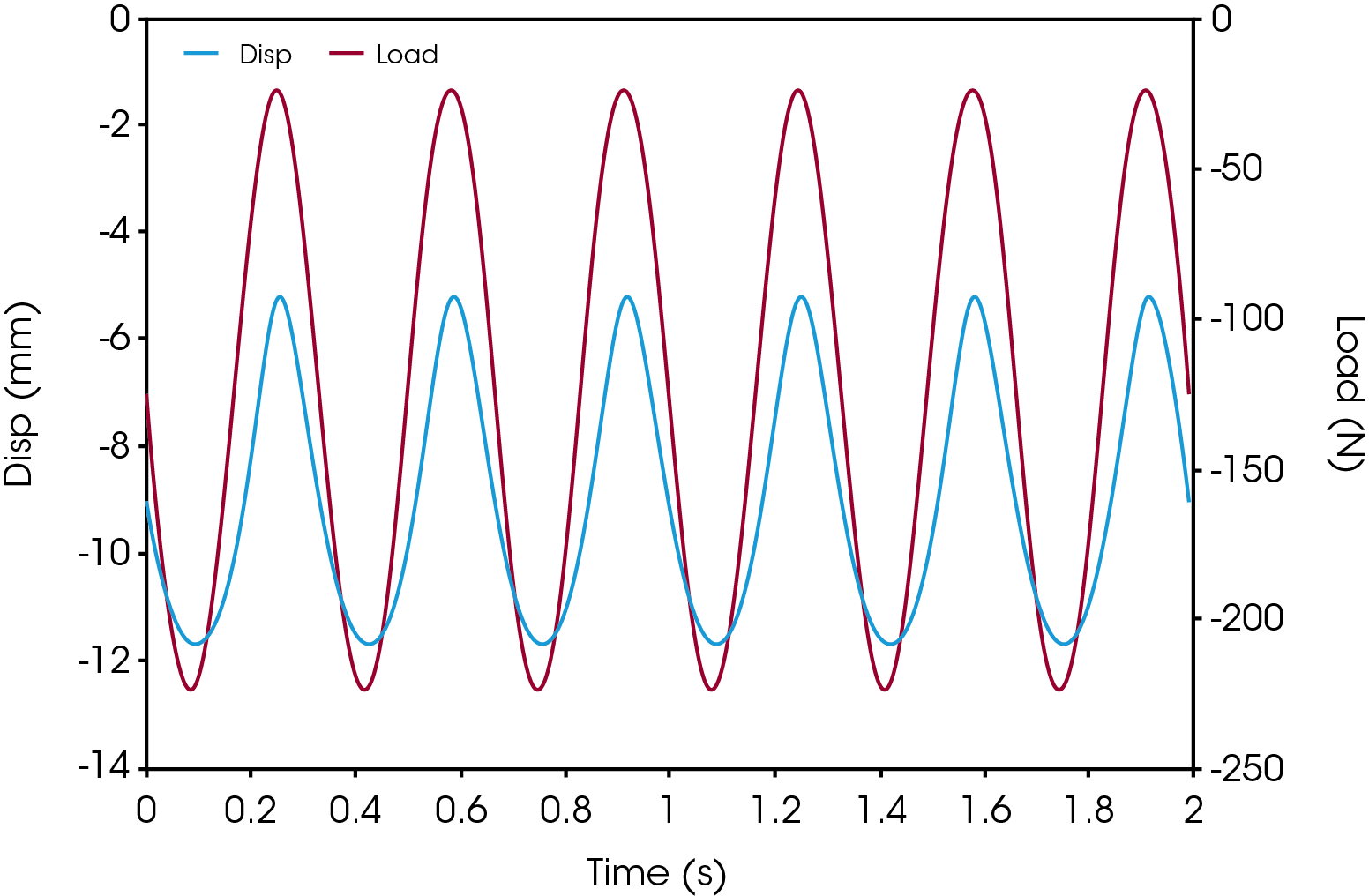
Applicable Test Standards
- ISO 14607 Non active surgical implants — Mammary implants— Particular requirements
- ISO 37 Rubber, vulcanized or thermoplastic — Determination of tensile stress-strain properties
- ISO 34-1 Rubber, vulcanized or thermoplastic — Determination of tear strength — Part 1: Trouser, angle and crescent
test pieces - ASTM F703 Standard Specification for Implantable Breast Prostheses
- ASTM F2051 Specification for Implantable Saline Filled Breast Prosthesis
- ASTM D412 Test Methods for Vulcanized Rubber and Thermoplastic Elastomers Tension
- ASTM D624 Standard Test Method for Tear Strength of Conventional Vulcanized Rubber and Thermoplastic Elastomers
| Model | 3310-ES | 3300-ES | 3510 |
|---|---|---|---|
| Force Capacity | 1,000 N | 3,000 N | 7,500 N |
| Stroke | 175 mm | 175 mm | 50 mm |
| Stroke Accuracy | 5 μm | 5 μm | 5 μm |

References
- International Society of Aesthetic Plastic Surgeons. 2018 International Survey on Aesthetic/Cosmetic Procedures. https://www.isaps.org/wp-content/uploads/2020/10/ISAPS-Global-Survey-Results-2018-1.pdf
- Saline, Silicone Gel, and Alternative Breast Implants Guidance for Industry and Food and Drug Administration Staff, Document 1239, USFDA, Sept 29, 2020. https://www.fda.gov/media/71081/download
Acknowledgement
Click here to download the printable version of this application note.

