Products | Microcalorimeters | TA Instruments RS-DSC
TA Instruments RS-DSC
The Next Generation of Thermal Stability Testing for Biologic Drugs
The TA Instruments RS-DSC (Rapid Screening-Differential Scanning Calorimeter) is a powerful and versatile instrument that revolutionizes the thermal stability testing of biologic drugs with its high efficiency and streamlined analysis.

The TA Instruments RS-DSC Can Help
- Accelerate thermal stability testing: The TA Instruments RS-DSC provides detailed insights into drug product thermal stability significantly quicker by analyzing up to 24 samples simultaneously. The TA Instruments RS-DSC’s technology also simplifies characterization of highly concentrated drug products.
- Improve efficiency: The TA Instruments RS-DSC ensures materials are used efficiently by utilizing disposable MFCs (Micro Fluidic Chips) to contain the sample. MFCs require <15 ul sample volume, and the design facilitates a cleaner, more streamlined operation by reducing the need for tedious sample dilution, repetitive instrument cleaning, and contamination risks.
- Make more informed decisions: NanoAnalyze™ Software effortlessly handles the increased data volume produced by the TA Instruments RS-DSC, providing in-depth, precise insights about a molecule’s thermal stability and thermodynamic properties.

Groundbreaking Capabilities
- Enhanced Throughput: The TA Instruments RS-DSC enables the analysis of 24 samples simultaneously which significantly speeds up research and accelerates biologic drug market entry.
- Resource Efficiency: The TA Instruments RS-DSC’s minimal sample volume requirements help ensure maximum material use and minimize cost.
- High Concentration Proficiency: The TA Instruments RS-DSC excels in testing an extensive range of sample concentrations and has a unique capacity for analyzing very high concentration drug products efficiently and effectively.
- Simplified Workflow: The TA Instruments RS-DSC streamlines operations by removing the need for sample dilution when working with high-concentration samples, and the disposable microfluidic chips reduce or eliminate the need for cleaning and reduce the risk of contamination.
- Comprehensive Data Analysis: NanoAnalyze Software manages data and provides detailed insights to optimize development.
Features & Benefits
- Parallel Analysis: Unique high throughput analysis enables up to 24 simultaneous measurements to accelerate research.
- Single-Use Microfluidic Technology: MFCs streamline operation by simplifying the characterization of high concentration drug products, as well as reducing cleaning time and contamination risks.
- State-of-the-Art Data Analysis Software: The robust and user-friendly NanoAnalyze Software automatically and consistently analyzes data for in-depth and rapid evaluation.
| RS-DSC | |
| Cell Geometry | Disposable microfluidic |
| Cell Material | Glass |
| Sample Format | MFC (Micro Fluidic Chips) |
| Working cell volume | 11 µL |
| Sample Capacity | 24 MFCs |
| Typical Sample Concentration | 20 mg/mL – 330+ mg/mL IgG (protein dependent)1 |
| Sample throughput | > 96 samples/day |
| Temperature Range | 20-100 °C |
| Temperature Scan Rate | 1 or 2 °C/min |
| Temperature accuracy | ± 0.2 °C (across all calorimeters); ± 0.1 °C reproducibility2 |
2 Using DPPC in water at pH 7 at 1 °C/min Accelerate research, improve efficiency, and make more informed decisions with the TA Instruments RS-DSC, a revolutionary new high-throughput thermal stability testing instrument specifically designed for biologic drug development.
Technology
Groundbreaking Capabilities
Microfluidic Technology: The Future of Precision and Convenience
Equipped with cutting-edge MFCs (Micro Fluidic Chips), the TA Instruments RS-DSC is designed to contain the sample effortlessly. This technological integration eliminates the need for repetitive cleaning of the instrument measurement cell between runs, saving time, reducing the risk of contamination, and enabling more precise and reliable readings.
The MFCs are disposable and enhance operational ease, enabling quick transitions and safeguarding the instrument from hazardous substances. The novel MFC design exemplifies cutting-edge, low-volume, single-use technology, facilitating ease of sample loading and preparation with standard lab equipment. A sample can be prepared, sealed, and ready for analysis in less than one minute, requiring only minimal volumes for precise evaluation.
Watch Product Demonstration
Applications
The TA Instruments RS-DSC is ideal for a wide range of applications in biologic drug development, including:
Thermal stability is a leading indicator of overall clinical success of a biologic drug product, and DSC (Differential Scanning Calorimetry) is a primary tool that is used to characterize the effect of the solution environment on protein stability. Effects on protein stability can be reflected in small shifts in Tmax or in changes of up to tens of degrees resulting from altering variables such as pH, buffer, ionic strength, excipients, and detergents on protein stability.
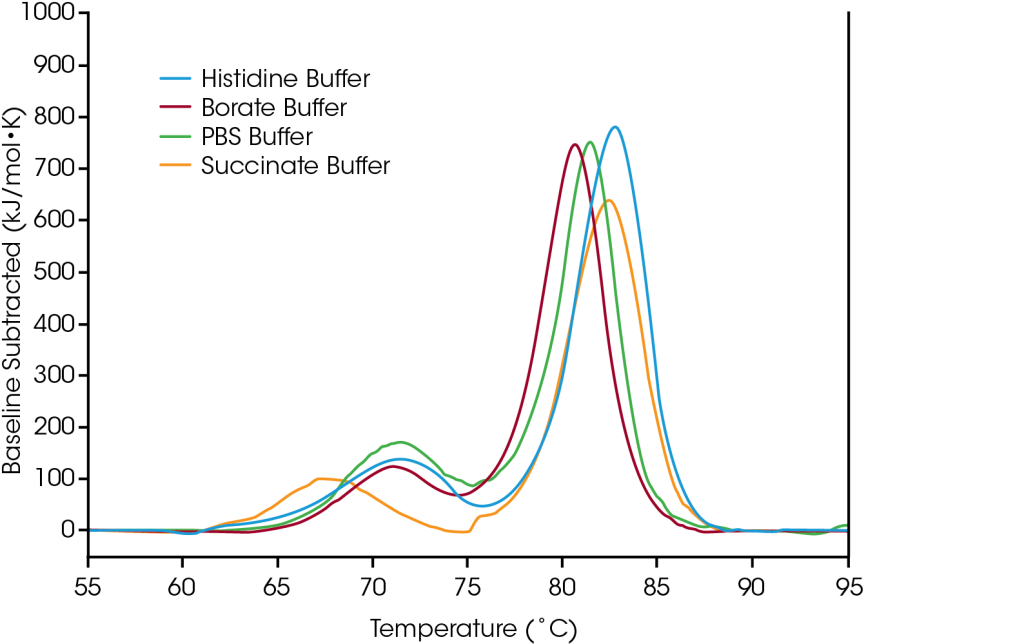
To demonstrate how the data from formulation screening can aid in selection of buffer components, the antibody trastuzumab was tested in four common buffer conditions: 1) A common working buffer (PBS), 2) A lysine-conjugation enabling buffer for the synthesis of labeled antibody for cellular trafficking studies or drug conjugation (borate), 3) A trastuzumab-based antibody drug conjugate (succinate), and 4) The native formulation buffer for trastuzumab (histidine).
The first unfolding event, corresponding to unfolding of the CH2 domain is not significantly impacted by the histidine, borate, or PBS buffers. However, the succinate buffer destabilizes the CH2 domain resulting in lowering the onset of unfolding and Tmax,1 by about 3°C. With respect to the main transition reflecting the Fab and CH3 unfolding events, the histidine and succinate buffers are the most stabilizing, with a Tmax,2 of 82.66°C. The main transition is least stable in the borate buffer with a Tmax,2 of 80.69°C. Unsurprisingly, the most stabilizing buffer formulation for trastuzumab in this sample set is the histidine buffer used for final formulation of the approved drug product.
Protein mutations are a common strategy for optimizing protein structure and function, and even single amino acid modifications can have a measurable effect on overall protein stability. Using DSC (Differential Scanning Calorimetry) to aid in characterization of engineered protein modifications is essential for understanding the structural impact of the mutation on the protein as a whole and can help guide decision making in the biologic drug development pipeline. To demonstrate the types of effects sequence modification can have on stability, a small panel engineered proteins were screened for changes in thermal stability resulting from single amino acid mutations in the protein sequence.
In the parent, unfolding occurs within one major thermal transition at a Tmax of 75.92°C. A single amino acid mutation was made which has no major effect on short-term thermal stability (Mutation 1); however, alternative single amino acid mutations are shown to have significant impacts on the protein’s stability (Mutation 2 and Mutation 3). As illustrated in the significant destabilization in Mutation 3, modification does not always have the same effect, rather it depends on both the site of the modification and on physicochemical properties of the new amino acid. Optimizing the desired functional benefits of sequence modification with the structural stability of the protein as a whole aids in the comprehension of the structure-function relationship and can facilitate development of advanced therapeutics.
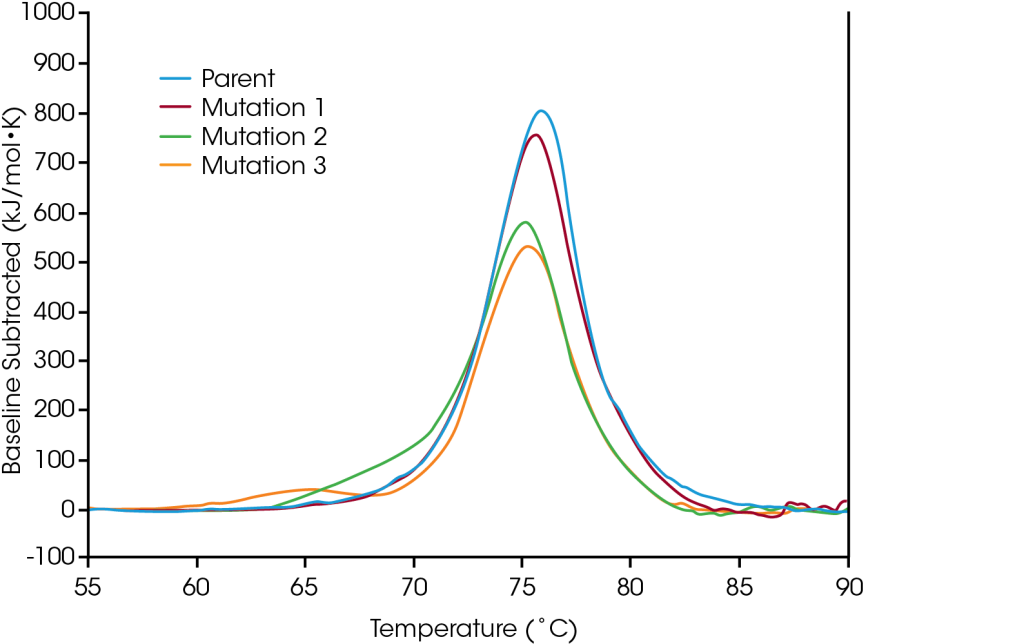
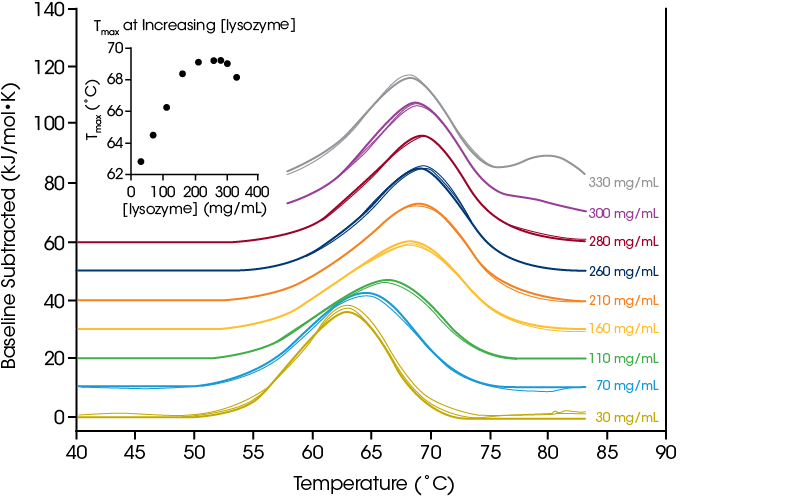
The TA Instruments RS-DSC is uniquely designed to handle high concentration biologic drug samples, with a specialized focus on antibody drugs and antibody drug conjugates. With the growing success of antibody therapeutics, the pharmaceutical industry has increased interest in high concentration dosage forms that enable subcutaneous and ocular drug delivery. As such, formulations with concentrations of 50 – 150 mg/mL antibody are common and can be as high as 200+ mg/mL. Formulating proteins at high concentrations can increase susceptibility to physical instability. Conversely, some cases studies have shown enhancement in thermal stability at increased concentrations Thus, understanding of thermal unfolding and response to the solution environment at the formulation concentration of interest is a critical metric for mitigating drug product liability.
To demonstrate the ability to test high concentration protein samples and illustrate the importance of testing at the desired formulation concentration, we evaluated chicken egg white lysozyme from 30 – 330 mg/mL in glycine buffer. With a simple single transition thermogram at low concentrations (~1 mg/mL), lysozyme is commonly used as a reference test sample for DSC. Through evaluation of protein concentrations up to 100-fold higher, we observed a concentration dependence on lysozyme stability.